Home > News > Can FGFR2b Antibody Become a New Option for Targeted Therapy in Gastric Cancer?
Can FGFR2b Antibody Become a New Option for Targeted Therapy in Gastric Cancer?
- Gastric cancer, a highly prevalent malignant tumor worldwide, presents a major challenge in optimizing treatment strategies.
Signaling PathwayFGFR2b antibodyGastric cancerGastric cancer treatment
Recent Advances
I. Why Does Gastric Cancer Treatment Require New Targeted Strategies?
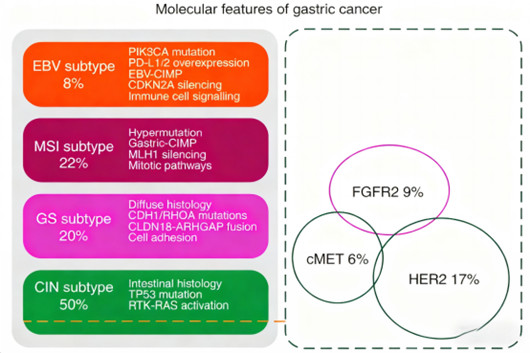
Gastric cancer, a highly prevalent malignant tumor worldwide, presents a major challenge in optimizing treatment strategies. Traditional chemotherapy regimens show limited efficacy in advanced gastric cancer patients, with median overall survival typically under one year and median progression-free survival only about six months. This therapeutic dilemma drives researchers to continuously explore new molecular targets and treatment strategies. In the molecular classification of gastric cancer, approximately 10% of patients exhibit FGFR2 gene amplification. This alteration does not overlap with HER2 amplification or cMET overexpression, representing a unique molecular subtype and providing a new direction for precision therapy.
FGFR2b, an isoform of FGFR2, plays a crucial role in tumorigenesis and progression. Studies show that aberrant activation of FGFR2b is closely associated with tumor proliferation, angiogenesis, and metastasis, and its overexpression is significantly correlated with poor patient prognosis. This discovery positions FGFR2b as a highly potential therapeutic target, particularly for patient subgroups that currently lack effective treatment options beyond chemotherapy.
II. What are the Characteristics of the FGFR2b Antibody's Mechanism of Action?
The FGFR2b antibody exerts its anti-tumor effects through a dual mechanism. Firstly, it specifically blocks the binding of endogenous ligands to the FGFR2b receptor, thereby inhibiting the abnormal activation of downstream signaling pathways. Research indicates that ligands like FGF7, FGF10, and FGF22 binding to FGFR2b can promote tumor cell proliferation and survival, and antibody intervention effectively blocks these biological effects.
Secondly, engineering optimization of the Fc segment significantly enhances Antibody-Dependent Cell-mediated Cytotoxicity (ADCC). Defucosylation allows the antibody to bind more effectively to the FcγRIIIa receptor on natural killer (NK) cells, thereby enhancing their tumor cell killing capacity. This dual mechanism not only directly inhibits tumor growth but also achieves a synergistic anti-tumor effect by activating the immune system.
III. What Potential Does Preclinical Research Demonstrate?
In preclinical studies, the FGFR2b antibody demonstrated encouraging anti-tumor activity. In the OCUM2 human gastric cancer xenograft model, monotherapy showed significant tumor growth inhibition. More notably, combination with standard chemotherapy regimens (5-fluorouracil and cisplatin) demonstrated synergistic effects, providing a solid theoretical foundation for subsequent clinical research.
The study also found that the therapeutic effect based on ADCC is closely related to the infiltration level of NK cells in the tumor microenvironment. The relatively high infiltration level of NK cells in gastric cancer tissue creates favorable conditions for the application of the FGFR2b antibody. Furthermore, the uniformity of FGFR2b expression distribution on the tumor cell surface might also influence the treatment effect; these factors require close attention in follow-up clinical studies.
IV. How are the Phase I Clinical Trial Results Evaluated?
Phase I clinical trial results provided important safety and efficacy data. During the dose escalation phase, administering the antibody every two weeks at doses ranging from 0.3-15 mg/kg revealed no dose-limiting toxicities or maximum tolerated dose, indicating a favorable safety profile. In the expansion cohort, patients were stratified into different groups based on FGFR2b expression levels to explore the correlation between biomarkers and treatment response.
Safety analysis showed that treatment-related adverse events occurred in 50.6% of patients, most of which were mild to moderate in severity. The most common adverse events included fatigue, nausea, and dry eye, the latter considered target-mechanism related. Notably, a few cornea-related adverse events were observed in patients receiving higher doses and longer treatment durations, but these were all reversible changes. The overall safety profile supports the further development of this antibody in subsequent studies.
V. How Do the Efficacy Data Compare to Other Treatment Options?
In patients with gastric cancer and gastroesophageal junction adenocarcinoma exhibiting high FGFR2b expression, the objective response rate reached 17.9%, with a median duration of response of 12.6 weeks. This data shows a significant advantage in the population of advanced, recurrent patients. Compared to other targeted therapies, anti-angiogenic drugs achieve an ORR of approximately 3.2% in similar populations, and immune checkpoint inhibitors show response rates between 12-16%.
Compared to another emerging target, the Claudin18.2 antibody, the FGFR2b antibody also demonstrates competitiveness. The Claudin18.2 antibody monotherapy achieved an ORR of 10%, increasing to 20% when combined with chemotherapy. It is important to note that different targeted therapies may be suitable for different patient subgroups, emphasizing the importance of biomarker-guided individualized treatment.
VI. What are the Future Directions and Challenges?
As research deepens, the FGFR2b antibody shows broad prospects in the field of gastric cancer treatment. The ongoing Phase III clinical trial will further verify its efficacy and safety in combination with standard chemotherapy. However, several key issues remain to be addressed, including the selection of optimal biomarkers, overcoming resistance mechanisms, and optimizing combination therapy strategies.
From a translational research perspective, further clarification of the FGFR2b expression level cutoff value and establishment of standardized detection methods are needed. Simultaneously, exploring rational combinations with other targeted drugs or immune checkpoint inhibitors may bring greater clinical benefits to patients. Additionally, more comprehensive strategies for preventing and managing treatment-related adverse events need to be developed.
Overall, the FGFR2b antibody represents a promising new option for targeted therapy in gastric cancer. Through precise patient selection and rational treatment strategies, this novel therapeutic approach holds new hope for gastric cancer patients with specific molecular subtypes. As more clinical data accumulates and research progresses, the FGFR2b antibody is poised to occupy an important position in the field of precision therapy for gastric cancer.
VII. Which Manufacturers Provide FGFR2b Antibodies?
Hangzhou Start Bio-tech Co., Ltd.'s self-developed "S-RMab® FGFR2b Recombinant Rabbit Monoclonal Antibody" is a high-performance antibody product characterized by high isoform specificity, excellent sensitivity, and exceptional staining consistency. This product is ideal for applications in companion diagnostics for targeted therapy, tissue development research, and precision medicine.
Product Core Advantages:
| Advantage Feature | Detailed Description |
| High Isoform Specificity & Clear Membrane Localization | Precisely recognizes the Fibroblast Growth Factor Receptor 2 isoform IIIb (FGFR2b), with very low cross-reactivity to other isoforms like FGFR2c. Demonstrates excellent cell membrane-specific staining in FFPE samples, with clear background and accurate localization, providing a reliable basis for precise interpretation. |
| Excellent Staining Stability & Batch Consistency | Under strict quality control standards, the product exhibits excellent staining stability and minimal batch-to-batch variation, ensuring high comparability of results across different laboratories and experimental batches, providing stable support for clinical companion diagnostics and translational research. |
Suitable Key Application Scenarios:
| Application Area | Specific Use |
| Companion Diagnostics for Targeted Therapy | For detecting FGFR2b protein expression in various solid tumors like gastric cancer and urothelial carcinoma, providing key basis for patient selection for FGFR2b-targeted drugs (e.g., bemarituzumab). |
| Tumor Differential Diagnosis & Subtyping | For identifying FGFR2b-positive tumors and assisting in molecular subtyping, exploring its relationship with clinicopathological features and prognosis. |
| Tissue Development & Epithelial Homeostasis Research | For studying the role of FGFR2b in embryonic development, tissue repair, and the maintenance of epithelial cell homeostasis. |
| Drug Development & Efficacy Prediction | Serves as a key tool antibody for evaluating the efficacy of FGFR2b-targeted drugs and developing biomarkers in preclinical research. |
Professional Technical Support: We provide detailed product technical documentation, including
complete IHC experimental protocols, optimized antigen retrieval methods, and professional interpretation guidance, fully committed to assisting customers in obtaining accurate and reliable results in precision medicine and targeted therapy research.
Hangzhou Start Bio-tech Co., Ltd. is always dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "S-RMab® FGFR2b Recombinant Rabbit Monoclonal Antibody" or to request a sample test, please feel free to contact us.
Product Information
| Catalog Number | Product Name | Product Parameters |
| S0B2231 | FGFR2b Recombinant Rabbit mAb (SDT-423-18) | Host : Rabbit Conjugation: Unconjugated |
| S0B2230 | FGFR2b Recombinant Rabbit mAb (SDT-423-2) | Host : Rabbit Conjugation: Unconjugated |
| S0B2359 | FGFR2b Recombinant Rabbit mAb (SDT-423-121) | Host : Rabbit Conjugation: Unconjugated |
| S0B2232 | S-RMab® FGFR2b Recombinant Rabbit mAb (SDT-423-33) | Host : Rabbit Conjugation: Unconjugated |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026
- How to Understand the Core Structure and Immune Functions of Ig Antibodies? 1/20/2026
- CD163 Antibody: How to Promote Vascular Regeneration and Homeostasis Recovery af 1/19/2026


