Home > News > Can CDK2 Inhibitors Overcome the Challenge of Drug Resistance in Cancer Therapy?
Can CDK2 Inhibitors Overcome the Challenge of Drug Resistance in Cancer Therapy?
- Cyclin-dependent kinases (CDKs), as crucial members of the serine/threonine kinase family, play a central role in cell cycle checkpoint regulation and DNA transcription.
CancerCDK2Cancer TherapyCyclin
Recent Advances
1. How Does Dysregulation of Cell Cycle Drive Tumor Initiation and Progression?
Cyclin-dependent kinases (CDKs), as crucial members of the serine/threonine kinase family, play a central role in cell cycle checkpoint regulation and DNA transcription. The catalytic activity of these kinases is precisely regulated by cyclins and CDK inhibitors, forming a key regulatory network controlling cell division and proliferation. Research indicates that family members such as CDK1, CDK2, CDK4, CDK6, and CDK7 are directly involved in regulating cell cycle transitions and division; their functional abnormalities are closely associated with the development and progression of various malignancies.
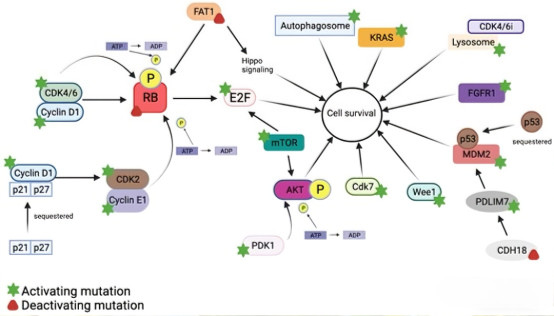
During the cell cycle, the binding of CDK4/6 to cyclin D initiates the phosphorylation of the retinoblastoma protein (Rb), leading to the release of E2F transcription factors and driving the transition from the G1 phase to the S phase. The subsequent binding of CDK2 to cyclin E further propels this process, ensuring normal cell cycle progression. However, this precisely regulated network is often disrupted in tumor cells, leading to uncontrolled cell proliferation and ultimately the formation of malignant tumors.
2. What Key Pathways Are Involved in the Resistance Mechanisms to CDK4/6 Inhibitors?
Although CDK4/6 inhibitors have achieved remarkable success in treating estrogen receptor-positive/HER2-negative breast cancer, becoming a standard therapy for this subtype, drug resistance remains a significant clinical challenge. Epidemiological data show that approximately 20% of patients exhibit primary resistance initially, while about 50% develop acquired resistance and disease progression within 25 months of treatment.
In-depth studies reveal that resistance to CDK4/6 inhibitors is associated with multiple molecular mechanisms. The most prominent among these is the compensatory activation of the CDK2 pathway. When CDK4/6 activity is inhibited, tumor cells upregulate CDK2 activity through cyclin E amplification and activation of the MYC transcription factor. The CDK2-cyclin E complex can serve as a compensatory pathway to continue Rb phosphorylation, release E2F transcription factors, and thus sustain tumor cell proliferative capacity. This mechanism has been experimentally validated in multiple CDK4/6-resistant cell lines.
3. What Strategic Value Does CDK2 Hold in Cancer Therapy?
As a key node in the cell cycle regulatory network, CDK2 demonstrates significant therapeutic value in overcoming resistance to CDK4/6 inhibitors. Research indicates that high expression of cyclin E is closely associated with reduced sensitivity to CDK4/6 inhibitors. Clinical data analysis shows that patients with high cyclin E expression have significantly shorter progression-free survival, suggesting this biomarker could serve as an important indicator for predicting the efficacy of CDK4/6 inhibitors.
From a therapeutic strategy perspective, simultaneously targeting CDK4/6 and CDK2 may achieve more comprehensive and sustained cell cycle arrest. This multi-target inhibition strategy aims to block compensatory activation pathways, potentially reducing the emergence of resistance and offering a new solution to overcome the limitations of current CDK4/6 inhibitors. Particularly in patients who have relapsed or progressed after CDK4/6 inhibitor therapy, CDK2 inhibition might restore tumor cell sensitivity to treatment.
4. What Preclinical Characteristics Do Novel CDK2/4/6 Inhibitors Exhibit?
New-generation CDK2/4/6 inhibitors have shown promising antitumor activity in preclinical studies. In vitro experiments, these compounds significantly inhibit the proliferation of various tumor cell lines. Notably, in preclinical models such as the HCC1806 breast cancer model, OVCAR3 ovarian cancer model, and MCF7 breast cancer model, they demonstrate effective tumor growth inhibition.
Regarding safety assessment, preclinical data suggest these novel inhibitors have a favorable safety profile. Their unique multi-target inhibitory properties might enhance efficacy through synergistic effects while reducing the risk of compensatory activation associated with single-target inhibition. These characteristics lay a solid foundation for subsequent clinical research and provide new avenues for addressing CDK4/6 inhibitor resistance.
5. What Opportunities and Challenges Does the Clinical Development of CDK2 Inhibitors Face?
The clinical development of CDK2 inhibitors faces significant opportunities alongside multiple challenges. From a patient needs perspective, there remains a substantial unmet clinical need for hard-to-treat tumors such as ER+/HER2- breast cancer that has relapsed or progressed after CDK4/6 inhibitor therapy, platinum-resistant high-grade serous ovarian carcinoma, and triple-negative breast cancer.
Regarding development strategy, rational clinical trial design and patient selection criteria are crucial. Patient stratification based on biomarkers, potentially using cyclin E expression levels as a key predictive indicator, may improve treatment response rates. Additionally, determining the optimal dosing regimen and combination strategies are critical considerations in clinical development. Balancing efficacy and safety to maximize the therapeutic benefit-risk ratio is a core challenge in the clinical development of CDK2 inhibitors.
6. What is the Future Direction for Multi-Target Inhibition Strategies?
With the deepening understanding of the cell cycle regulatory network, the development of multi-target CDK inhibitors is evolving. Beyond the CDK2/4/6 combination, researchers are exploring inhibition strategies targeting other CDK family members. These strategies aim to more comprehensively block cell cycle progression while minimizing the activation of compensatory pathways.
In terms of clinical application, the development of personalized treatment strategies is particularly important. Integrating genomic, transcriptomic, and proteomic information to build precise patient stratification models could enable more accurate treatment selection. Furthermore, exploring the combination of CDK inhibitors with other targeted agents or immune checkpoint inhibitors represents a significant future research direction.
As more clinical data accumulate and novel inhibitors are developed, CDK2-targeted therapy holds promise for providing new treatment options for cancer patients, particularly showcasing unique value in overcoming drug resistance and treating refractory tumors. These advances will further refine the cancer targeted therapy landscape and propel precision medicine to deeper levels.
7. Which Companies Provide CDK2 Antibodies?
Hangzhou Start Biological Technology Co., Ltd. independently developed the "CDK2 Recombinant Rabbit Monoclonal Antibody" (Product Name: CDK2 Recombinant Rabbit mAb (SDT-064-40), Catalog Number: S0B2152). This is a high-performance antibody product characterized by high specificity, excellent sensitivity, and outstanding staining consistency. Developed using recombinant rabbit monoclonal antibody technology and rigorously validated across multiple platforms including Immunohistochemistry (IHC) and Western Blot (WB), it holds significant application value in areas such as cell cycle regulation research, tumor proliferation activity assessment, and targeted therapy development.
Core Product Advantages:
- High Specificity & Clear Nuclear Localization: Precisely recognizes Cyclin-Dependent Kinase 2 (CDK2), demonstrating exceptional nuclear-specific staining in Formalin-Fixed Paraffin-Embedded (FFPE) samples with clear background and accurate localization, providing a reliable basis for precise interpretation.
- Excellent Staining Stability & Batch Consistency: Under stringent quality control standards, the product exhibits superior staining stability and minimal inter-batch variation, ensuring reliable and reproducible results under different experimental conditions, providing stable assurance for both clinical and basic research.
Suitable for Key Application Scenarios:
This product is an ideal tool for the following research areas:
- Cell Cycle & Proliferation Studies: Used to study cell cycle progression and proliferation regulation mechanisms as CDK2 is a key regulator of the G1/S phase transition.
- Tumor Malignancy Assessment: Used to evaluate the proliferative activity and malignancy degree of various cancers (e.g., breast cancer, lung cancer, colorectal cancer).
- CDK Inhibitor Research: Serves as a target protein for CDK inhibitor drugs, facilitating studies on drug mechanisms of action and efficacy evaluation.
- Development & Differentiation Research: Used in studies related to cell cycle regulation during embryonic development, tissue regeneration, and cell differentiation.
Professional Technical Support:
We provide detailed product technical documentation, including complete IHC experimental protocols, optimized antigen retrieval methods, and clear interpretation criteria, fully assisting customers in obtaining accurate and reliable results in cell cycle research and tumor pathology diagnosis.
Hangzhou Start Biological Technology Co., Ltd. is committed to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. To learn more about the "CDK2 Recombinant Rabbit Monoclonal Antibody" (Catalog Number S0B2152) or to request a sample for testing, please feel free to contact us.
Product Information
| Catalog Number | Product Name | Product Parameters |
| UA080212 | CDK2 Protein | Host : Human Expression System : E.coli Conjugation : Unconjugated |
| S0B0819 | CDK2 Recombinant Rabbit mAb (S-1308-13) | Host : Rabbit Conjugation : Unconjugated |
| S0B2152 | CDK2 Recombinant Rabbit mAb (SDT-064-40) | Host : Rabbit |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026
- How to Understand the Core Structure and Immune Functions of Ig Antibodies? 1/20/2026
- CD163 Antibody: How to Promote Vascular Regeneration and Homeostasis Recovery af 1/19/2026


