Home > News > How Do Phosphorylated Tau Antibodies Reveal the Mechanism of Protective Mutations in Alzheimer's Disease?
How Do Phosphorylated Tau Antibodies Reveal the Mechanism of Protective Mutations in Alzheimer's Disease?
- Alzheimer's disease (AD), as the most common neurodegenerative disorder, has its pathogenesis as a major research focus in neuroscience.
NeurosciencePhosphorylated Tau antibodyAlzheimer's diseaseNeuroscience
Recent Advances
I. What Key Questions Does Alzheimer's Disease Genetics Research Face?
Alzheimer's disease (AD), as the most common neurodegenerative disorder, has its pathogenesis as a major research focus in neuroscience. It can be classified into early-onset and late-onset based on the age of onset, both exhibiting typical pathological features like β-amyloid (Aβ) deposition and neurofibrillary tangles. In hereditary early-onset AD, mutations in the PSEN1, PSEN2, and APP genes are identified as deterministic causative factors. Carriers of these mutations typically develop the disease around age 40, with rapid progression. However, a groundbreaking case report in 2019 revealed a remarkable phenomenon: a patient carrying the PSEN1-E280A pathogenic mutation, who was also homozygous for the APOE3-R136S mutation (APOE3-Christchurch, APOE3ch), exhibited only mild cognitive decline in their 70s---delaying the expected onset by approximately 30 years.
This rare case garnered significant interest from researchers. Notably, despite having substantial Aβ deposits in the brain, the patient did not show significant cognitive dysfunction caused by tau pathology, suggesting a halt in the disease process at the Aβ stage. This finding indicated that the APOE3ch mutation might have a protective effect, but evidence from a single case was insufficient to establish causality, requiring deeper mechanistic studies to validate this hypothesis.
II. How Does the APOE3ch Mutation Affect the Alzheimer's Disease Pathological Process?
To investigate the specific mechanism of the APOE3ch mutation, researchers created a humanized APOE3ch knock-in mouse model and crossed it with APP/PS1 Aβ model mice. They then established a disease model simulating Aβ-induced tau pathology by intracerebrally injecting human tau extracts. Using specific phosphorylated Tau (p-Tau) antibodies for detection, researchers found that not only were the size and number of amyloid plaques significantly reduced in the brains of APOE3ch mice, but more importantly, the deposition of p-Tau around each Aβ plaque was also markedly decreased.
Further analysis revealed significantly reduced synaptic damage around plaques in the brains of APOE3ch mice, manifested as decreased BACE1-positive neurites and enhanced staining of synaptic markers synapsin and PSD95. These findings suggest that the APOE3ch mutation not only affects Aβ deposition but, more critically, attenuates the Aβ-induced spread of tau pathology and synaptic damage, which might be a key mechanism for its ability to delay disease progression.
III. What is the Specific Mechanism by Which APOE3ch Affects Tau Protein Metabolism?
To deeply explore the molecular mechanism by which APOE3ch influences tau metabolism, researchers conducted a series of in vitro experiments using bone marrow-derived macrophages. Through phagocytosis assays of pH-sensitive dye-labeled tau fibrils, they discovered that cells derived from APOE3ch mice exhibited significantly enhanced tau uptake capability. Further mechanistic studies indicated that this enhanced effect was related to altered interactions between APOE3ch and cell surface receptors.
APOE3ch has reduced binding affinity to receptors like LRP1 and HSPG compared to wild-type APOE3, thereby reducing competition with tau protein for binding to these receptors. This allows more tau protein to be recognized and phagocytosed by microglia. Furthermore, monitoring the post-phagocytosis degradation process showed a significant reduction in the amount of tau released from APOE3ch cells, indicating improved degradation efficiency. This enhanced phagocytosis-degradation capability might be a crucial mechanism for limiting the spread of tau pathology.
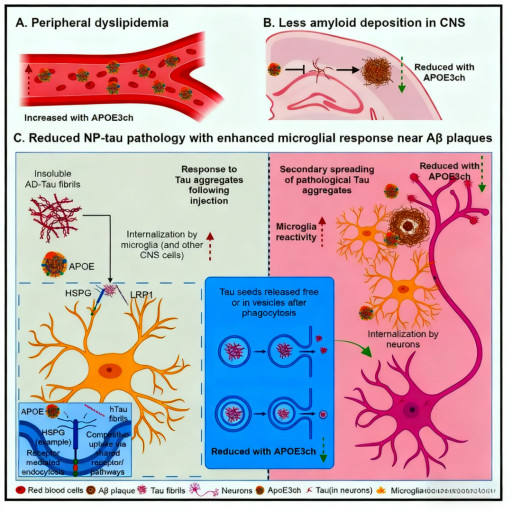
A key question was whether the protective effect of APOE3ch depends on the Aβ environment. Researchers injected human tau protein into mouse models lacking Aβ deposits and found that the inhibitory effect of APOE3ch on tau pathology was significantly weakened. This result suggests that the protective effect of APOE3ch might require the specific microenvironment provided by Aβ deposits.
Interestingly, even in the absence of Aβ, microglia from APOE3ch mice still exhibited enhanced phagocytic-lysosomal activity, but this level of activation might be insufficient to effectively control the spread of tau pathology. This indicates that Aβ deposits and altered microglial function might act synergistically in the protective mechanism, together forming a defense network that inhibits disease progression.
V. What are the Implications of This Discovery for Alzheimer's Disease Treatment?
This study systematically reveals, for the first time, the molecular mechanism by which the APOE3ch mutation delays Alzheimer's disease progression, providing important insights for developing new therapeutic strategies. The results suggest that interventions targeting the transition stage from Aβ to tau pathology could be an effective treatment strategy. Particularly, modulating microglial function to enhance their clearance capacity for tau protein, while avoiding excessive inflammatory responses, could become a direction for future drug development.
Phosphorylated Tau antibodies played a crucial role in this research, not only for detecting the extent and distribution of tau pathology but also helping to elucidate the specific stages at which APOE3ch influences tau metabolism. These findings not only deepen our understanding of AD pathogenesis but also provide an important basis for the development of biomarkers and the identification of therapeutic targets.
As the understanding of the APOE3ch protective mechanism deepens, it may be possible in the future to develop novel therapies that mimic its mode of action to delay or even halt the progression of Alzheimer's disease. Concurrently, these studies also suggest a need to pay closer attention to the interactions between Aβ and tau pathology, and the regulatory role of microglia in this process.
VI. Which Manufacturers Provide Phosphorylated Tau Antibodies?
Hangzhou Start Bio-tech Co., Ltd.'s self-developed "Tau (phospho T181) Recombinant Rabbit Monoclonal Antibody" is a neuropathology research antibody characterized by ultra-high phosphorylation specificity, exceptional affinity, and excellent batch consistency. This product is ideal for applications in biomarker detection for Alzheimer's disease and other tauopathies, disease mechanism research, and drug evaluation.
Product Core Advantages:
| Advantage Feature | Detailed Description |
| Ultra-High Phosphorylation Specificity & Sensitivity | Rigorously validated across multiple platforms including phosphorylated/non-phosphorylated peptide ELISA, Western Blot, and immunohistochemistry, this product demonstrates exceptional specific recognition for Tau pT181 with extremely high sensitivity, effectively distinguishing phosphorylated Tau in the pathological state of AD from normal Tau protein. |
| Excellent Stability & Batch Consistency | Under a strict production and quality control system, the product exhibits excellent stability with minimal intra-batch and inter-batch variation, ensuring comparability and reproducibility of data across different experiments, providing stable support for large cohort studies and clinical trials. |
Suitable Key Application Scenarios:
| Application Area | Specific Use |
| Alzheimer's Disease Biomarker Detection | For detecting pT181-Tau levels in cerebrospinal fluid (CSF) and plasma samples, serving as an important biomarker for the early and differential diagnosis of AD. |
| Neuropathological Diagnosis & Research | For the pathological identification and quantitative analysis of brain tissue sections from patients with AD and other tauopathies. |
| Disease Mechanism Exploration | For investigating the role of pT181-Tau in neurofibrillary tangle formation, tau pathology propagation, and its contribution to neuronal dysfunction. |
| Drug Development & Efficacy Evaluation | Serves as a key pharmacodynamic biomarker for evaluating the effect of tau-targeted therapies on specific phosphorylation sites in preclinical and clinical trials. |
Professional Technical Support: We provide detailed product technical documentation, including complete specificity validation data, application protocols for body fluid samples like CSF/plasma, and professional technical consultation, fully committed to assisting customers in making breakthroughs in neuroscience and precision medicine.
Hangzhou Start Bio-tech Co., Ltd. is always dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "Tau (phospho T181) Recombinant Rabbit Monoclonal Antibody" or to request a sample test, please feel free to contact us.
Product Information
| Catalog Number | Product Name | Product Parameters |
| S0B1195 | Tau (phospho T212/S214) Recombinant Rabbit mAb (S-1538-74) | Host : Rabbit Conjugation : Unconjugated |
| S0B3505 | Tau (phospho T205) Recombinant Rabbit mAb (SDT-2034-37) | Host : Rabbit Conjugation : Unconjugated |
| S0B3156 | Tau (phospho T181) Mouse mAb (SDT-200-5) | Host : Mouse Conjugation : Unconjugated |
| S0B3099 | Tau (phospho T181) Recombinant Rabbit mAb (SDT-R045) | Host : Rabbit |
| S0B0029 | Tau (phospho T181) Recombinant Rabbit mAb (SDT-R045) | Host : Rabbit |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026
- How to Understand the Core Structure and Immune Functions of Ig Antibodies? 1/20/2026
- CD163 Antibody: How to Promote Vascular Regeneration and Homeostasis Recovery af 1/19/2026


