- Mouse TGF-β ELISA Kit

- Product Detail
- Company Profile
Product Specification
| Usage | Required experimental equipment: 1. Microplate reader (450nm) 2. High-precision pipettes and pipette tips: 0.5-10uL, 5-50uL, 20-200uL, 200-1000uL 3. 37°C incubator 4. Distilled or deionized water Sample handling and requirements: The detection range of the kit is not equivalent to the concentration range of the analyte in the sample. Before the experiment, it is recommended to estimate the analyte concentration in the sample based on relevant literature and conduct preliminary experiments to determine the actual concentration in the sample. If the analyte concentration in the sample is too high or too low, dilute or concentrate the sample appropriately. If the sample being tested is not listed in the instructions, it is recommended to conduct a preliminary experiment to verify the validity of the test. Serum: Incubate whole blood samples collected in serum separator tubes at room temperature for 2 hours or at 4°C overnight, then centrifuge at 1000×g for 20 minutes. Remove the supernatant, or store at -20°C or -80°C, but avoid repeated freezing and thawing. Plasma: Collect specimens using EDTA or heparin as an anticoagulant and centrifuge at 1000×g for 15 minutes at 2-8°C within 30 minutes of collection. Remove the supernatant for analysis, or store at -20°C or -80°C, but avoid repeated freezing and thawing. Tissue homogenate: Rinse tissue with pre-chilled PBS (0.01M, pH 7.4) to remove residual blood (lysed red blood cells in the homogenate may affect the measurement results). Weigh and mince the tissue. Add the minced tissue to the appropriate volume of PBS (generally a 1:9 weight-to-volume ratio, e.g., 1g of tissue sample to 9mL of PBS. The specific volume can be adjusted according to experimental needs and recorded. It is recommended to add protease inhibitors to the PBS) in a glass homogenizer and grind thoroughly on ice. To further lyse tissue cells, the homogenate can be sonicated or repeatedly freeze-thawed. Finally, centrifuge the homogenate at 5000×g for 5-10 minutes and remove the supernatant for analysis. Cell culture supernatant: Centrifuge at 1000×g for 20 minutes and remove the supernatant for analysis. Alternatively, store the supernatant at -20°C or -80°C, but avoid repeated freeze-thaw cycles. Other biological specimens: Centrifuge at 1000×g for 20 minutes and remove the supernatant for analysis. Sample Appearance: The sample should be clear and transparent, and suspended matter should be removed by centrifugation. Sample Storage: Samples collected for testing within one week can be stored at 4°C. If testing cannot be performed promptly, aliquot the sample into single-use portions and store at -20°C (for testing within one month) or -80°C (for testing within six months). Avoid repeated freezing and thawing. Hemolysis of the sample can affect the final test results, so hemolyzed samples are not suitable for this test. Sample Activation:  3. Preparation of biotinylated detection antibody working solution: 15 minutes before use, centrifuge the concentrated biotinylated antibody at 1000×g for 1 minute. Dilute 100× concentrated biotinylated antibody to a 1× working concentration with universal diluent (e.g., 10 μL concentrate + 990 μL universal diluent) and use on the same day. 4. Preparation of Enzyme Conjugate Working Solution: 15 minutes before use, centrifuge 100 μL of concentrated enzyme conjugate at 1000 × g for 1 minute. Dilute the 100 μL concentrated HRP enzyme conjugate with universal diluent to a 1 μL working concentration (e.g., 10 μL concentrate + 990 μL universal diluent). Use the same day. 5. Preparation of 1 μL Wash Solution: Dissolve 10 mL of 20 μL Wash Solution in 190 mL of distilled water. (Crystallization may occur in the concentrated wash solution after removal from the refrigerator. This is normal. Allow to stand at room temperature until the crystals have completely dissolved before preparation.) Procedure: 1. After equilibration at room temperature for 10 minutes, remove the desired strips from the aluminum foil bag. Seal the remaining strips in a ziplock bag and return them to 4°C. 2. Sample Addition: Add 100 μL of sample or standard of varying concentrations to the corresponding wells. Add 100 μL of universal diluent to the blank wells. Cover with film and incubate at 37°C for 1 hour. (Recommendation: Dilute the test samples at least 1-fold with universal diluent before adding them to the plate. This will minimize matrix effects on test results. When calculating sample concentrations, multiply by the dilution factor. It is recommended to run replicates for all test samples and standards.) 3. Biotinylated Antibody Addition: Remove the plate, discard the liquid, and do not wash. Add 100 μL of biotinylated antibody working solution directly to each well. Cover with film and incubate at 37°C for 1 hour. 4. Wash: Discard the liquid and add 300 μL of 1x wash buffer to each well. Let stand for 1 minute, shake off the wash buffer, and pat dry on absorbent paper. Repeat this process three times (a plate washer can also be used). 5. Add enzyme conjugate working solution: Add 100 μL of enzyme conjugate working solution to each well, cover with a film sealer, and incubate at 37°C for 30 minutes. 6. Wash: Discard the liquid and wash the plate five times according to the washing method in step 4. 7. Add substrate: Add 90 μL of substrate (TMB) to each well, cover with a film sealer, and incubate at 37°C in the dark for 15 minutes. 8. Add stop solution: Remove the ELISA plate and add 50 μL of stop solution directly to each well. Immediately measure the OD value of each well at a wavelength of 450 nm. Calculation of Experimental Results: Result Interpretation: 1. Calculate the average OD value of the standard and sample replicates and subtract the blank OD value as the correction value. Plot the standard curve of the four-parameter logistic function on double-logarithmic graph paper, with concentration as the horizontal axis and OD value as the vertical axis. 2. If the sample OD value is higher than the upper limit of the standard curve, dilute the sample appropriately and retest. Multiply the sample concentration by the corresponding dilution factor. 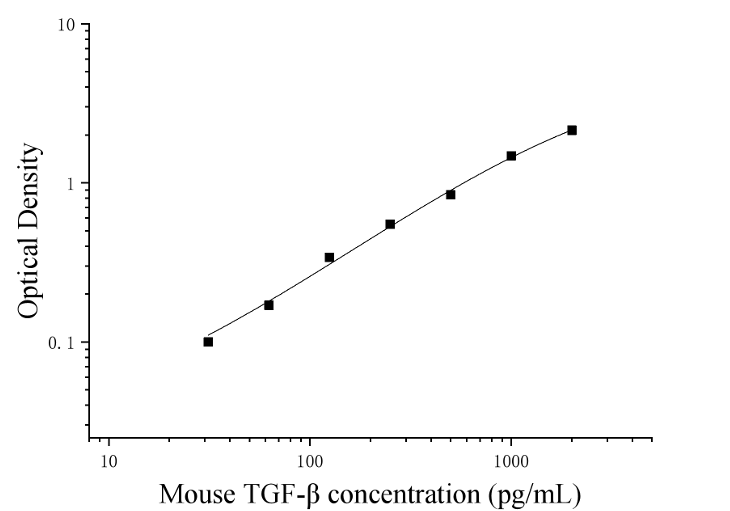 Kit Performance: 1. Repeatability: Intra-plate coefficient of variation (CV) less than 10%, inter-plate coefficient of variation (CV) less than 10%. 2. Recovery: Mouse TGF-beta was spiked into serum, plasma, and cell culture supernatant of healthy mice at three different concentrations, and the recovery rate was calculated.
| ||||||||||||||||||||||||||||||||||||||
| Sensitivity | 15.3 pg/mL | ||||||||||||||||||||||||||||||||||||||
| Theory | This kit uses a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Sample, standard, biotin-labeled detection antibody, and HRP conjugate are sequentially added to microwells pre-coated with mouse transforming growth factor β (TGF-β) capture antibody. After incubation and washing, the sample is developed using the substrate TMB. TMB is converted to blue by HRP peroxidase and then to yellow by acid. The intensity of the color is positively correlated with the amount of mouse transforming growth factor β (TGF-β) in the sample. The absorbance (OD) is measured at 450 nm using a microplate reader to calculate the sample concentration. | ||||||||||||||||||||||||||||||||||||||
| Source | Mouse | ||||||||||||||||||||||||||||||||||||||
| Synonym | Mouse TGF-β ELISA Kit | ||||||||||||||||||||||||||||||||||||||
| Detection Type | Double antibody sandwich method | ||||||||||||||||||||||||||||||||||||||
| Composition |
| ||||||||||||||||||||||||||||||||||||||
| Background | Transforming growth factor β (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily, which includes three distinct mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, and TGFB3) and numerous other signaling proteins. TGFB proteins are produced by all leukocyte lineages. Activated TGF-β complexes with other factors to form a serine/threonine kinase complex that binds to the TGF-β receptor. The TGF-β receptor is composed of type 1 and type 2 receptor subunits. Upon TGF-β binding, type 2 receptor kinase phosphorylates and activates type 1 receptor kinase, thereby initiating a signaling cascade. This leads to the activation of various downstream substrates and regulatory proteins, inducing the transcription of diverse target genes and playing a role in differentiation, chemotaxis, proliferation, and activation of many immune cells. TGF-β is secreted by many cell types, including macrophages, in a latent state, complexed with two other polypeptides: latent TGF-β binding protein (LTBP) and latent associated peptide (LAP). Serum proteases such as plasmin catalyze the release of active TGF-β from the complex. This typically occurs on the surface of macrophages, where the latent TGF-β complex binds to CD36 via its ligand, thrombospondin-1 (TSP-1). Inflammatory stimuli that activate macrophages enhance the release of active TGF-β by promoting the activation of plasma proteins. Macrophages can also internalize latent TGF-β complexes bound to IgG secreted by plasma cells, and then release active TGF-β into the extracellular fluid. One of its primary functions is to regulate inflammatory processes, particularly in the intestine. TGF-β also plays a key role in stem cell differentiation and T cell regulation and differentiation. Due to its role in immunity and stem cell regulation and differentiation, it is a cytokine that has been extensively studied in the fields of cancer, autoimmune diseases, and infectious diseases. The TGF-β superfamily includes endogenous growth inhibitory proteins; increased TGF-β expression is often associated with the malignancy of many cancers and a defect in the cellular growth inhibitory response to TGF-β. Dysregulation of its immunosuppressive function has also been implicated in the pathogenesis of autoimmune diseases, although its effects are mediated by the milieu of other cytokines present. | ||||||||||||||||||||||||||||||||||||||
| General Notes | 1. Strictly adhere to the specified incubation time and temperature to ensure accurate results. All reagents must be at room temperature (20-25°C) before use. Refrigerate reagents immediately after use. 2. Improper plate washing may result in inaccurate results. Ensure that all liquid in the wells is aspirated thoroughly before adding substrate. Do not allow the wells to dry out during incubation. 3. Remove any residual liquid and fingerprints from the bottom of the plate, as this will affect the OD value. 4. The substrate developer solution should be colorless or very light in color. Do not use substrate solution that has turned blue. 5. Avoid cross-contamination of reagents and specimens to prevent erroneous results. 6. Avoid direct exposure to strong light during storage and incubation. 7. Do not expose any reagents to bleaching solvents or the strong fumes emitted by bleaching solvents. Any bleaching agent will destroy the biological activity of the reagents in the kit. 8. Do not use expired products, and do not mix components with different product numbers and batches. 9. Recombinant proteins from sources other than the kit may not be compatible with the antibodies in this kit and will not be recognized. 10. If there is a possibility of disease transmission, all samples should be managed properly and samples and testing devices should be handled according to prescribed procedures. | ||||||||||||||||||||||||||||||||||||||
| Storage Temp. | If the unopened kit is stored at 4°C, the shelf life is 6 months. | ||||||||||||||||||||||||||||||||||||||
| Test Range | 31.25-2000 pg/mL |
-
AntBio is a biotechnology group company dedicated to serving life sciences, aiming to help scientists accelerate research and improve work efficiency. AntBio provides comprehensive and high-quality reagent tools for basic research, drug development, and diagnosis, including research grade antibodies, proteins, biochemical reagents, and assay kits. These research tools are widely used in different segments of life science research. The group company currently consists of three brands, Absin, Starter-Bio and UA-Bio.
| Request Information |
| Other Products |
| Related Products |
| Recently viewed products |
- SiteMap



