Reagents:
Molecular Biology
Biochemistry
Cell Biology
ELISA / Diagnostic Kits
Antibody
Serum/Medium
Other Reagents
- Human MMP-9 ELISA Kit

- Product Detail
- Company Profile
Product Specification
| Usage | Need to bring your own test equipment 1. Microplate reader (can measure the absorption value of 450nm detection wavelength and the absorption value of 540nm or 570nm correction wavelength) 2. High-precision liquid dispenser and disposable tip 3. Distilled water or deionized water 4. Bottle washing (spray bottle), multi-channel plate washer or automatic plate washer 5. 500mL measuring cylinder 1. Preparation before the experiment 1. Sample collection and storage ① Cell culture supernatant: particulate matter should be removed by centrifugation; Test the sample immediately. If the sample is not tested in time after collection, it is recommended to pack it according to the one-time usage amount and store it in a refrigerator at-20 ℃ to avoid repeated freezing and thawing. Samples may need to be diluted with diluent (1 ×). ② Serum: Use a serum separation tube (SST) to collect samples, and place the samples at room temperature for 30 minutes. Centrifuge for 15 minutes at a rotation speed of 1000 g. The serum was removed immediately and tested immediately. If the sample is not tested in time after collection, it is recommended to pack it according to the one-time usage amount and store it in a refrigerator ≤-20 ℃ to avoid repeated freezing and thawing. Samples may need to be diluted with diluent (1 ×). ③ Plasma: Plasma was collected using EDTA, heparin or citric acid as anticoagulant, centrifuged for 15 minutes within 30 minutes after collection, rotated at 1000g, and detected immediately. If the sample is not tested in time after collection, it is recommended to pack it according to the one-time usage amount and store it in a refrigerator ≤-20 ℃ to avoid repeated freezing and thawing. Samples may need to be diluted with diluent (1 ×). 2. Reagent preparation (Please place all reagents and samples at room temperature before use and let them stand15Minutes. All experimental samples and standards are recommendedDo repeat hole detection) ① Preparation of 1 × washing liquid: The concentrated washing liquid in the kit is 20 × mother liquid, which needs to be diluted into 1 × working liquid with distilled water before use.Example:Take 10mL of concentrated washing solution + 190mL of distilled water and make the volume to 200mL. In actual operation, the amount used can be calculated first, and then prepared. ② Preparation of 1 × dilution buffer: The concentration and dilution buffer in the kit is 10 × mother liquor, which should be diluted to 1 × working solution with distilled water before use.Example:Make up to 30 mL with 3 mL of concentration and dilution buffer + 27 mL of distilled water. In actual operation, the required amount of dilution buffer solution can be calculated according to the sample dilution factor, and then prepared. ③ Antibody detection: centrifuge the dry powder to the bottom of the tube, dissolve it with 110uL of dilution buffer (1 ×), and let it stand at room temperature for 5 minutes to obtain 100 × mother liquor; Dilute to 1 × working solution before use. Calculate the required volume according to the dosage of 100uL per well.Example:After 10 wells were used, 10 uL of the detection antibody having a working concentration of 100 times was taken, and the volume was diluted to 1 mL using a dilution buffer (1 ×) to obtain 1 mL of the detection antibody having a working concentration of 1 ×. ④ SA-HRP: SA-HRP is 40 × mother liquor, which needs to be diluted with dilution buffer (1 ×) before use to prepare 1 × working solution, and the required amount per well is 100uL.Example:After 10 wells were used, 25 uL of 40 × mother liquor + 975 uL of dilution buffer (1 ×) was diluted to 1 mL to obtain 1 mL of detection antibody having a 1 × working concentration. ⑤ Color development solution: According to 100uL per hole, calculate the dosage required for the current test, take out the corresponding volume of color development solution, and protect it from light; The chromogenic solution removed is for the same day only. ⑥ Standard: The freeze-dried standard is re-dissolved with dilution buffer (1 ×), and the re-dissolving volume is 1000uL to obtain the standard mother liquor with a concentration of 2000pg/mL. Gently shake for at least 5 minutes and it dissolves well. 300 uL of dilution buffer (1 ×) was added to each dilution tube. Make serial dilutions of the standard mother liquor according to the figure below, and each tube must be thoroughly mixed before pipetting to the next tube. The standard mother solution without dilution can be used as the highest point of the standard curve (2000 pg/mL), and the dilution buffer (1 ×) can be used as the zero point of the standard curve (0 pg/mL). 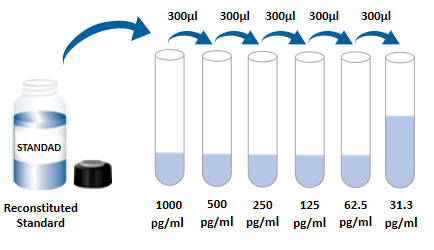 2. Operation steps 1. Prepare all required reagents and standards; 2. Take out the microplate from the sealed bag that has been balanced to room temperature. Please put the unused slats back into the aluminum foil bag and reseal them; 3. Add 300uL of washing liquid to the microplate, let it stand and soak for 30 seconds, discard the washing liquid and pat the microplate dry on absorbent paper. Please use it immediately and do not let the microplate dry; 4. Add different concentration standards, experimental samples or quality control products to the corresponding wells, 100uL per well. Sealing the reaction hole with plate sealing adhesive paper and incubating at room temperature for 2 hours; 5. Suck off the liquid in the plate and wash the plate with a bottle washer, a multi-channel plate washer or an automatic plate washer. 300 uL of washing solution was added to each well, and then the washing solution in the plate was aspirated off. Repeat the operation 3 times. Trying to absorb the residual liquid as much as possible every time you wash the plate will help to get good experimental results. At the end of the last plate washing, please suck all the liquid in the plate or turn the plate upside down, and pat all the residual liquid dry on absorbent paper; 6. Add 100 uL of detection antibody to each well. Seal the reaction wells with plate sealing tape and incubate at room temperature for 2 hours; 7. Repeat the plate washing operation in step 5; 8. Add 100uLSA-HRP to each well and incubate at room temperature for 20 minutes. Be careful to avoid light; 9. Repeat the plate washing operation in step 5; 10. Add 100uL of chromogenic solution to each microwell, incubate at room temperature for 5-30 minutes, and avoid light; 11. Add 50uL of stop solution to each microwell, and the color of the solution in the well will change from blue to yellow. If the color of the solution changes to green or the color changes are inconsistent, pat the microplate gently to mix the solution evenly; 12. Within 30 minutes after adding the stop solution, measure the absorbance value of 450nm using a microplate reader, and set 540nm or 570nm as the calibration wavelength. If dual-wavelength correction is not used, the accuracy of the results may be affected; 13. Calculation Results: Average the corrected absorbance values (OD450-OD540 or OD570), multiple well readings for each standard and sample, and then subtract the average zero standard OD value. Four-parameter logic (4-PL) curve fitting was performed using computer software to create the standard curve. Alternatively, a curve can be generated by plotting the logarithm of the standard concentration versus the logarithm of the corresponding OD value, and the best fit line can be determined by regression analysis. This process can generate a data fit that is sufficiently useful but less accurate. If the sample is diluted, the concentration should be calculated by multiplying the dilution factor.  3. Kit parameters 1. Recovery: Different levels of Human MMP-9 were spiked into cell culture medium samples and the recovery rate was determined. The recoveries ranged from 85 to 104%, with an average recovery of 97%. 2. Sensitivity: The lowest measurable dose (MDD) of Human MMP-9 is generally 0.156 ng/mL. The lowest measurable value is the corresponding concentration calculated from the mean of the zero-point absorbance values of 20 standard curves plus two standard deviations. 3. Calibration: This ELISA kit was calibrated with high purity recombinant Human MMP-9 protein expressed by E. coli. 4. Linearity: 4 different samples were spiked with high concentrations of Human MMP-9, and then the samples were diluted to the detection range with diluent (1 ×) to determine their linearity.
5. Specificity: This ELISA method can detect natural and recombinant human MMP-9 protein propeptide with a molecular weight of 92kDa and activated form of MMP-9 protein with a molecular weight of 82kDa, but cannot detect MMP-9 protein with a molecular weight of 65kDa. The following factors were configured with diluent (1 ×) at concentrations of 100 ng/mL or 200 ng/mL to detect cross-reaction with Human MMP-9. Interference with Human MMP-9 was detected by incorporating 200 ng/mL of the interfering factor into the mid-range recombinant Human MMP-9 standard. No significant cross-reactivity or interference was observed.
4. Analysis of frequently asked questions 1. Whiteboard (after the color development is completed, no color appears)
2. Flower plate (blank and negative positive controls are normal, but the OD value of specimen wells is obviously higher)
5. Experimental flow chart  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species Reactivity | Human | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Theory | This kit adopts double antibody sandwich enzyme-linked immunosorbent detection technology. Specific anti-human MMP-9 antibodies were pre-coated on high affinity labeled plates. The standard substance, the sample to be tested and the biotinylated detection antibody are added to the well of the enzyme label plate, and after incubation, the MMP-9 present in the sample binds to the solid phase antibody and the detection antibody to form an immune complex. After washing to remove unbound material, horseradish peroxidase-labeled Streptavidin-HRP was added. After washing, a chromogenic substrate is added to protect the color from light. A stop solution was added to stop the reaction, and the absorbance value was measured at a wavelength of 450 nm (reference calibration wavelength of 540 nm or 570 nm). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonym | 92 kDa gelatinase, 92 kDa type IV collagenase, CLG4B, EC 3.4.24, EC 3.4.24.35, Gelatinase B, GELB, macrophage gelatinase, MANDP2, matrix metallopeptidase 9, matrix metalloproteinase 9, matrix metalloproteinase-9, MMP9, MMP-9, type V collagenase | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Composition | Please use within the expiration date of the kit
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Background | Matrix metalloproteinases (MMPs), also known as matrixins, are members of the zinc-calcium-dependent proteolytic enzyme family, which can break down the extracellular matrix (ECM) and participate in the processing of various molecules in different subcellular environments. MMPs play important functions in numerous physiological processes, such as embryonic development, morphogenesis, reproduction, and tissue remodeling. Also involved in inflammation as well as autoimmune diseases such as arthritis, cancer and cardiovascular diseases. The amount of newly synthesized MMPs is mainly regulated at the transcriptional level, and the proteolytic activity of already existing MMPs is controlled not only by the activity of the zymogen, but also by the inhibition of enzyme activity by endogenous inhibitors, such as α2-macroglobulin, and tissue inhibitors of metalloproteinases (TIMPs). MMP-9 (also known as gelatinase B, 92 kDa type IV collagenase, 92 kDa gelatinase, type V collagenase) is secreted as a proglycosylation enzyme. Activation of the zymogen involves proteolytic removal of the N-terminal precursor region, resulting in the formation of an active enzyme of 82 kDa. Active human MMP-9 has 72% and 74% amino acid sequence homology with mouse and rat MMP-9, respectively. In addition to the zinc binding site, the catalytic domain contains three consecutive fibronectin type II homologous units that bind to gelatin. A proline-rich hinge region links the catalytic domain to the C-terminal heme-like domain. In vitro experiments, after treatment with 4-aminophenyl ethyl acetate (APMA), the enzymogen can not only produce active enzyme, but also produce a C-terminal truncated form with equivalent activity. MMP-9 can degrade components in ECM, especially with very high specificity for denatured collagen (gelatin). MMP-9 also cleaves collagen types III, IV, V, XI, as well as elastin, nestin-1, and vitronectin. MMP-9 can also cleave a variety of chemokines and growth factors (e.g., IL-1β, CXCL8/IL-8, CXCL7, CXCL4, CXCL1, LatentTGF-β, membrane-bound TNF-α, VEGF, and FGFbasic), beta-amyloid, substance P, myelin basic protein. This action may increase or decrease the biological activity of these soluble factors, and may also cause them to be released from association with the ECM. MMP-9 can also trigger signaling pathways through various membrane proteins or induce them to fall off the cell membrane to inhibit signaling pathways (E.g. CD44, E-cadherin, integrin, ICAM-1 and IL-2Rα). MMP-9 is produced by a variety of normal and transformed cells, including neutrophils, monocytes, macrophages, astrocytes, fibroblasts, osteoclasts, chondrocytes, keratinocytes, endothelial cells, and epithelial cells. It affects physiological and pathological angiogenesis and vascular remodeling. Activated neutrophils release the MMP-9 precursor and do not bind to TIMP-1, allowing pro-angiogenic FGF-2 to be released from the ECM. The complex of MMP-9 and TIMP-1 failed to induce the release of FGF-2. Neutrophil-derived MMP-9 can exacerbate the inflammatory response, inducing the release of additional neutrophil MMP-9 by the production of collagen-derived polypeptides. MMP-9 also plays an important role in bone formation and remodeling, methamphetamine-induced behavioral sensitization and response, regulation of neuronal synaptic remodeling, trophoblast invasion during implantation, and activation of serine protease inhibitor α1. MMP-9-mediated shedding of adhesion molecules also has a direct effect on tumor cell invasion. Circulating levels of MMP-9 are elevated in many inflammatory disorders, including the formation of intraluminal thrombus, atherosclerosis, Crohn's disease, hepatitis C virus infection, colorectal cancer, and Duchenne muscular dystrophy. The ratio of MMP-9 to TIMP-1 was elevated in multiple sclerosis sera and cystic fibrous sputum but decreased in cytomegalovirus-infected sera. The level of free MMP-9 and the level of MMP-9 and lipocalin-2/NGAL complex were elevated in the urine of patients with ovarian cancer and patients with uterine urethral tract infection, respectively. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Notes | 1. Please use the kit within the validity period. 2. The components of different kits and different batch kits cannot be mixed. 3. If the sample value is greater than the highest value of the standard curve, the sample should be diluted with diluent (1 ×) and re-tested; If the cell culture supernatant sample needs to be distributed and diluted, cell culture medium can be used for other intermediate dilutions except dilution with diluent in the last step. 4. Differences in test results can be caused by a variety of factors, including the operation of the experimenter, the use of the pipette, the plate washing technique, the reaction time or temperature, the storage of the kit, etc. 5. The terminating solution in the kit is an acidic solution. Please protect your glasses, hands, face and clothes when using it. 6. For scientific research only, not for in vitro diagnosis. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Temp. | Kit unopened, stored at 2-8 °C. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Test Range | 31.3pg/mL-2000pg/mL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
bio-equip.cn
-
AntBio is a biotechnology group company dedicated to serving life sciences, aiming to help scientists accelerate research and improve work efficiency. AntBio provides comprehensive and high-quality reagent tools for basic research, drug development, and diagnosis, including research grade antibodies, proteins, biochemical reagents, and assay kits. These research tools are widely used in different segments of life science research. The group company currently consists of three brands, Absin, Starter-Bio and UA-Bio.
| Request Information |
| Other Products |
| Related Products |
| Recently viewed products |
- SiteMap



