Reagent Arrival Handling:1. Endometrial organoid culture mediumA can be stored at 4 ℃ for 3 months. It is recommended to store at 4 ℃ after receiving the goods. It is recommended to use it within 1 month. It is recommended to store it at-20 ℃ for a long time to avoid repeated freezing and thawing more than 2 times。
2、Tissue preservation solutionEPrimary tissue digestive juice,C contains nutrients to maintain cell activity. In order to maintain the activity of the nutrients of the reagent, it is not recommended to store it at-20 ℃ for a long time to avoid repeated freezing and thawing more than 2 times。 Operation steps and methods:1 tissue pretreatment1.1 Experimental materialsPrimary buffer B (pre-cooled at 4 ℃), tissue preservation solution E, sampling tube, tissue transport box and ice pack should be prepared in advance. 1.2 Acquisition and transportation of organizationsTissue sampling and transportation is the first and most easily neglected link in the successful construction of organoids. If the tissue is not preserved properly in the early stage, it will lead to problems such as poor cell activity, pollution, and few normal cells, which will reduce the success rate of organoid construction. The tissue should be washed 3-5 times in tissue preservation solution E and primary buffer solution B within 30 minutes of isolation, the blood on the tissue surface should be washed clean, put into tissue preservation solution E, and the sampling tube should be stored and transported at 4 ℃ at low temperature (within 72 hours). Primary culture of organoids 2 (using 24-well plates as an example)2.1 Experimental materialsPrimary buffer B (4 ℃), primary tissue digestion juice C (37 ℃), Matrigel (melted in a 4 ℃ refrigerator 24 hours in advance), endometrial organoid culture medium A (room temperature or 37 ℃), tweezers (10 ㎝), pointed ophthalmic surgical scissors/blade, disposable 60mm culture dish, 1.5 mL/15mL/50mL centrifuge tube, 100 μ cell filter, 3mL pasteurization pipet/1000ul pipette gun, 24-well cell culture plate, metal ice box, water bath. 2.2 Endometrial organoid construction2.2. 1 Treatment of the organizationThe collected endometrial tissue is recommended to be stored and transported at 2-8 ℃, and quickly transported to a clean laboratory for the experimental procedure of endometrial organoid construction, taking photos and registering detailed information. 2.2. 1.1 Cleaning of tissuesAfter the sampling tube is disinfected, take out the tissue on the ultra-clean table, put it in a Petri dish, add primary buffer B, blow and clean with a 3mL pasteurization pipette or a 1000ul pipette gun, and repeat the cleaning operation three times or more. 2.2. 1.2 Dissociation and digestion of tissuesRemove tissue impurities with ophthalmic scissors or surgical blade, transfer forceps to 1.5 mLEP tube, and further mechanically dissociate tissue with ophthalmic scissors to a volume of about 1 ~ 3mm3The tissue block was transferred to a 15 mLEP tube, and 5 mL of primary tissue digestion juice was added and digested by shaking at 37 °C for 15-25 minutes. Digestion process Observe the tissue digestion under a microscope every 10 minutes, take a small amount of digestive juice and observe under a microscope. After observing more cell clusters or single cells below 70μm, proceed to the next operation. The degree of tissue digestion is shown in Figure 1. If the amount of tissue is too small or the biopsy tissue is used1mLPrimary tissue digestive juiceCIn1.5 mL EPTube digestion. 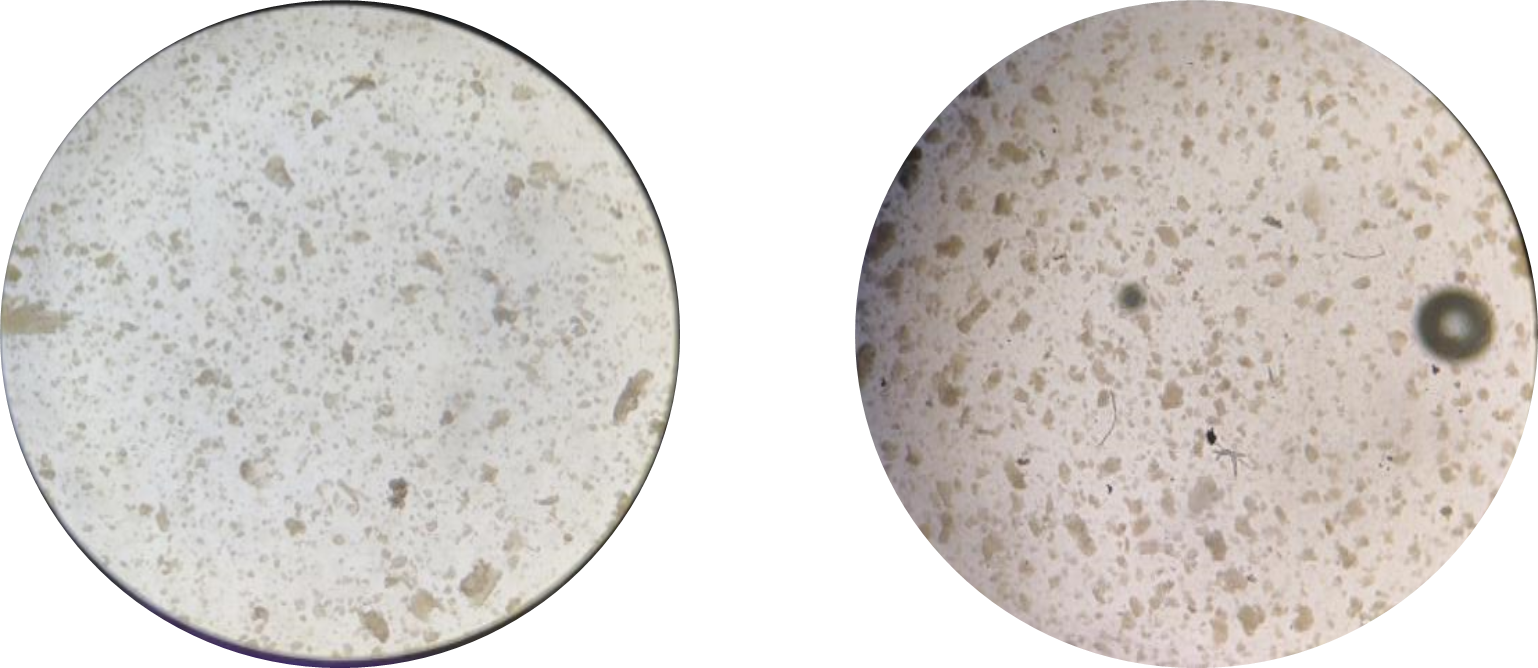
Figure 1 Tissue digestion into more cell clusters or more single cells 2.2. 1.3 Filtering of tissuesAfter the digestion is completed, the tissue vortexer is vortexed for 10 s, 3 times the volume of primary buffer B is added to terminate the digestion, the digested tissue mixture is filtered through a cell screen with a pore size of 100 μm, and the tissue is continuously rinsed with 10-20 ml of primary buffer B to collect more tissue mass cells, and the supernatant is discarded after enriching and centrifuging at 300g for 5 minutes; The centrifugation pellet was resuspended with 8-10 ml of primary buffer B (more impurities were removed), and the supernatant was discarded after 300 g of enrichment centrifugation for 5 minutes. If the cell pellet contains red blood cells, add 1-2mL of red blood cell lysate for 1-2min, dilute to 10mL, enrich 300g and centrifuge for 5min, and then discard the supernatant.Organoid culture was carried out directly when there were too little or no red blood cells precipitated. 2.2. 1.4 Organoid cultureObserve the volume of cell pellets collected by centrifugation, add 25 times the volume of Matrigel to resuspend, form a 3D culture space structure, and avoid bubbles during resuspension. The volume of cell pellet is shown in Figure 2. 300ul, 250ul, 150ul, and 100ul Matrigel are added according to the volume of cell pellet as shown in the figure. Figure 2 Cell pellet volume The 24-well cell culture plate is dispensed according to 25ul-30ul/well,Matrigel is maintained throughout0-4℃Operation under conditions。 Place the cell culture plate in a 37 ℃ incubator for 10-15 minutes. After the matrigel coagulates, add 750μl of human endometrial organoid medium A (room temperature) per well of the 24-well cell culture plate and place it in a 37 ℃ incubator for culture. Subculture of organoid 3 (using 24-well plate as an example)3.1 Experimental materialsPassage buffer G (4 ℃), organoid digestive juice D (room temperature or 37 ℃), Matrigel (melted in a 4 ℃ refrigerator 24 hours in advance), endometrial organoid medium A (room temperature or 37 ℃), 1.5 mL/15 mL centrifuge tube, 24-well cell culture plate, ice box. 3.2 Organoid passageSelect suitable organoids for passage, which usually grows for about one week. More than 20 organoids, or organoids with a size of 100-200 μ, can be seen under a microscope of 10X. Aspirate the medium, add an equal volume of passage buffer G to each well, gently blow the Matrigel with a pipette gun, collect it in a 15 mL centrifuge tube, transfer it to a centrifuge tube every 6-8 wells, and stand at 4 °C for 10-15 minutes. 3.2. 1 Organoid digestionAccording to the growth of organoids, it is decided whether digestive passage is needed.After centrifugation, if there is little sediment at the bottom of the tube, no cells are seen, and the matrigel is not stratified, it can be resuspended again, the centrifugal force is increased, and centrifuged again。 When the number of organoids is insufficient or the volume is small, centrifuge 300g for 5 minutes and discard the supernatant. When the number of organoids is large or the volume is large, centrifuge 300g for 5 minutes to discard the supernatant, and digestive juice digestion or mechanical digestion can be selected. Digestive juice digestion: Add 1-2mL of organoid digestive juice D, blow away the cell pellet, digest at room temperature for 2-4min, blow every minute, 20 times each time, and observe under a microscope until the digestion reaches (Figure A-B) state can be stopped. Digestion was terminated by adding 3 times the volume of organoid digest fluid in passage buffer G, and 300 G was centrifuged for 5 minutes.
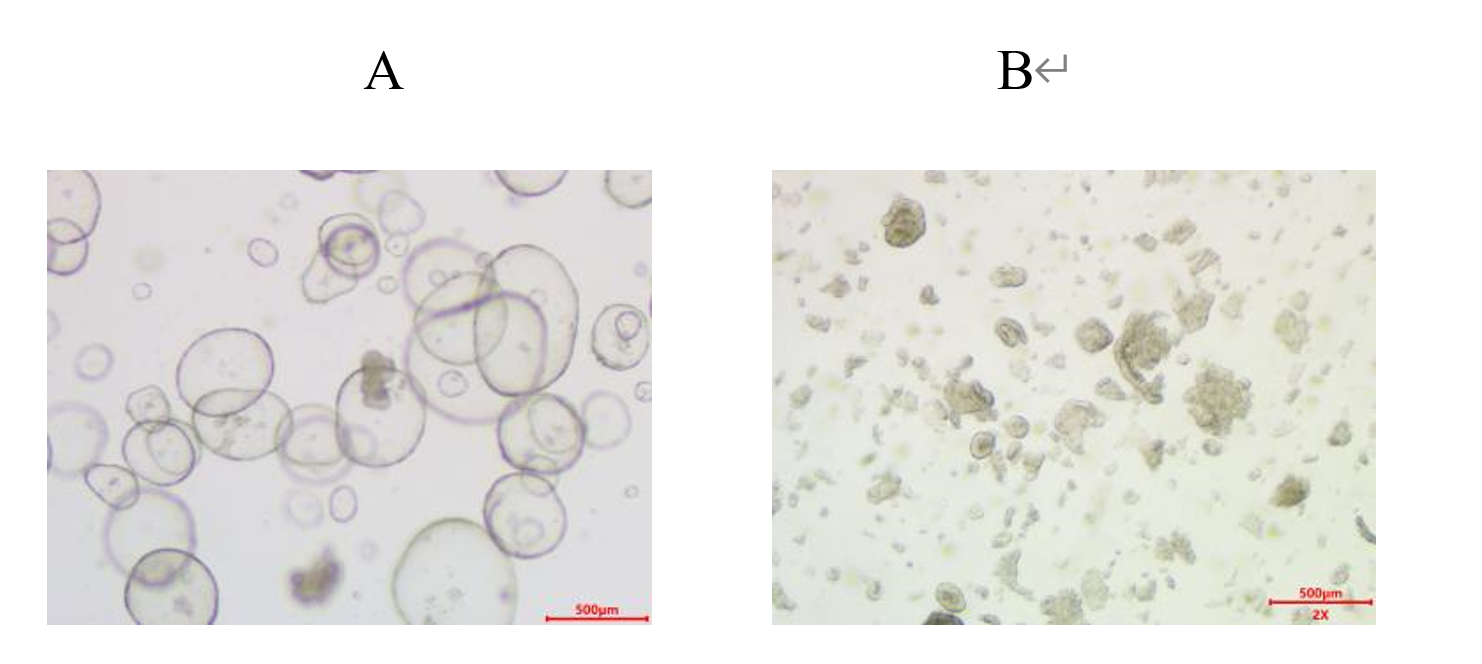 Figure Diagram of passage digestion degree of three types of organs 3.2. 2 Passage organoid cultureObserve the volume of organoid precipitate collected by centrifugation. If there is little precipitate, reserve 1 time the precipitate volume of supernatant for dispensing. If a lot of precipitate supernatant can be aspirated. Add 25 times the matrigel volume of organoid precipitate to resuspend organoids. For the volume of matrigel, please refer to "Organoid Primary Culture Operation Figure 2". The 24-well cell culture plate is dispensed according to 25ul-30ul/well,Matrigel is maintained throughout0-4℃Operation under conditions。 Place the cell culture plate in a 37 ℃ incubator for 10-15 minutes. After the matrigel coagulates, add 750μl of human endometrial organoid medium A (room temperature) per well of the 24-well cell culture plate and place it in a 37 ℃ incubator for culture. Cryopreservation of 4 organoids (using 24-well plates as an example)4.1 Experimental materialsPassage buffer G (4 ℃), organoid cryopreservation solution F (4 ℃), 15mL centrifuge tube, cell cryopreservation tube, program cooling box, pipette gun. 4.2 Organoid CryopreservationOrganoids that are not in use temporarily should be frozen and stored in a low temperature environment. Aspirate the medium, add an equal volume of passage buffer G to each well, gently blow the Matrigel with a pipette gun, collect it in a 15 mL centrifuge tube, transfer it to a centrifuge tube every 6-8 wells, and stand at 4 °C for 10-15 minutes. Centrifuge at 300g for 5 minutes to discard the supernatant, add 2mL of organoid cryopreservation solution F every three wells, gently pipetting and mix well, and transfer to cell cryopreservation tubes, each tube contains 1mL. Make the mark information, put it in the program cooling box, move it to a-80 ℃ refrigerator, and store it in a liquid nitrogen tank after 48 hours. Or put it in a 4 °C refrigerator for 40 minutes, put it in a-20 °C refrigerator for 2 hours, move it to a-80 °C refrigerator, and put it in a liquid nitrogen tank for 48 hours. 5 organoid resuscitation culture (using 24-well plate as an example)5.1 Experimental materialsPassage buffer G, endometrial organoid medium A, Matrigel (melted in a 4 °C refrigerator 24 hours in advance), 24-well cell culture plate, ice box, 15 mL centrifuge tube, water bath pan, 3 mL pasteurization pipette/pipette gun. 5.2 Organoid resuscitation cultureTake out the frozen organoids from the low temperature environment and quickly put them in a 37 ℃ water bath to thaw. During the water bath thawing process, the cryopreservation tube should be gently shaken to ensure that the cryopreservation solution is completely thawed in a short time. The thawed organoids were quickly transferred to a 15mL centrifuge tube, gently pipetted with a pipette gun for 6-8 times, and centrifuged at 300g for 5min to discard the supernatant; Add an appropriate amount of passage buffer G to resuspend, transfer it into a 1.5 mL centrifuge tube 300g and centrifuge for 5 minutes, and discard the supernatant. Add 120ul Matrigel to each cryopreservation tube for resuspension, dispense 24-well cell culture plates according to 25ul-30ul/well, and maintain the Matrigel at 0-4 ℃ throughout the process. Place the cell culture plate in a 37 ℃ incubator for 10-15 minutes. After the matrigel coagulates, add 750μl of human endometrial organoid medium A (room temperature) per well of the 24-well cell culture plate and place it in a 37 ℃ incubator for culture. 6 Matrigel useMatrigel was thawed overnight at ambient conditions of 2-8 °C. When using Matrigel, keep it in an ice box to prevent premature setting. The matrigel formed a gel at 37 °C within 20 minutes. 6.1 Matrigel characteristics:1. 4 ℃ can still maintain good fluidity for 14 consecutive days
2. Put it in a 37 ℃ incubator for 10-15min to solidify
3. It is not easy to break during the culture process, and the glue is removed to clean the non-stick culture plate
Scope of application:For Laboratory Research Use Only. Not For Use In Diagnostic Procedures. | 






