| Usage | Equipment required for the experiment:
1. Microplate reader (450nm)
2. High-precision pipette and pipette tips: 0.5-10uL, 5-50uL, 20-200uL, 200-1000uL
3. 37℃ constant temperature box
4. Distilled water or deionized water
Sample processing and requirements:
Serum: Place the whole blood sample collected in the serum separation tube at room temperature for 2 hours or at 4℃ overnight, then centrifuge at 1000×g for 20 minutes and take the supernatant. Alternatively, store the supernatant at -20℃ or -80℃, but avoid repeated freezing and thawing.
Plasma: Collect the specimen using EDTA or heparin as an anticoagulant. Centrifuge the specimen at 1000 × g for 15 minutes at 2-8°C within 30 minutes of collection. The supernatant can be assayed or stored at -20°C or -80°C, but avoid repeated freezing and thawing.
Tissue homogenization: Rinse the tissue with pre-chilled PBS (0.01M, pH 7.4) to remove residual blood (lysed red blood cells in the homogenate will affect the measurement results). Weigh the tissue and mince it. Add the minced tissue to the appropriate volume of PBS (generally a 1:9 weight-to-volume ratio, e.g., 1 g of tissue sample to 9 mL of PBS. The specific volume can be adjusted according to experimental needs and recorded. It is recommended to add protease inhibitors to the PBS) in a glass homogenizer and grind thoroughly on ice. To further lyse tissue cells, the homogenate can be sonicated or repeatedly frozen and thawed. Finally, centrifuge the homogenate at 5000×g for 5-10 minutes, and collect the supernatant for analysis.
Cell Lysis Buffer: Gently wash adherent cells with pre-chilled PBS, then trypsinize and collect the cells by centrifugation at 1000×g for 5 minutes. Suspension cells can be collected directly by centrifugation. Wash the collected cells three times with pre-chilled PBS and resuspend them in 150-200 μL of PBS per 1×10^6 cells (it is recommended to add protease inhibitors to the PBS; if the cell count is very low, reduce the PBS volume appropriately). Disrupt the cells by repeated freeze-thaw cycles or sonication. Centrifuge the extract at 1500×g for 10 minutes at 2-8°C, and collect the supernatant for analysis.
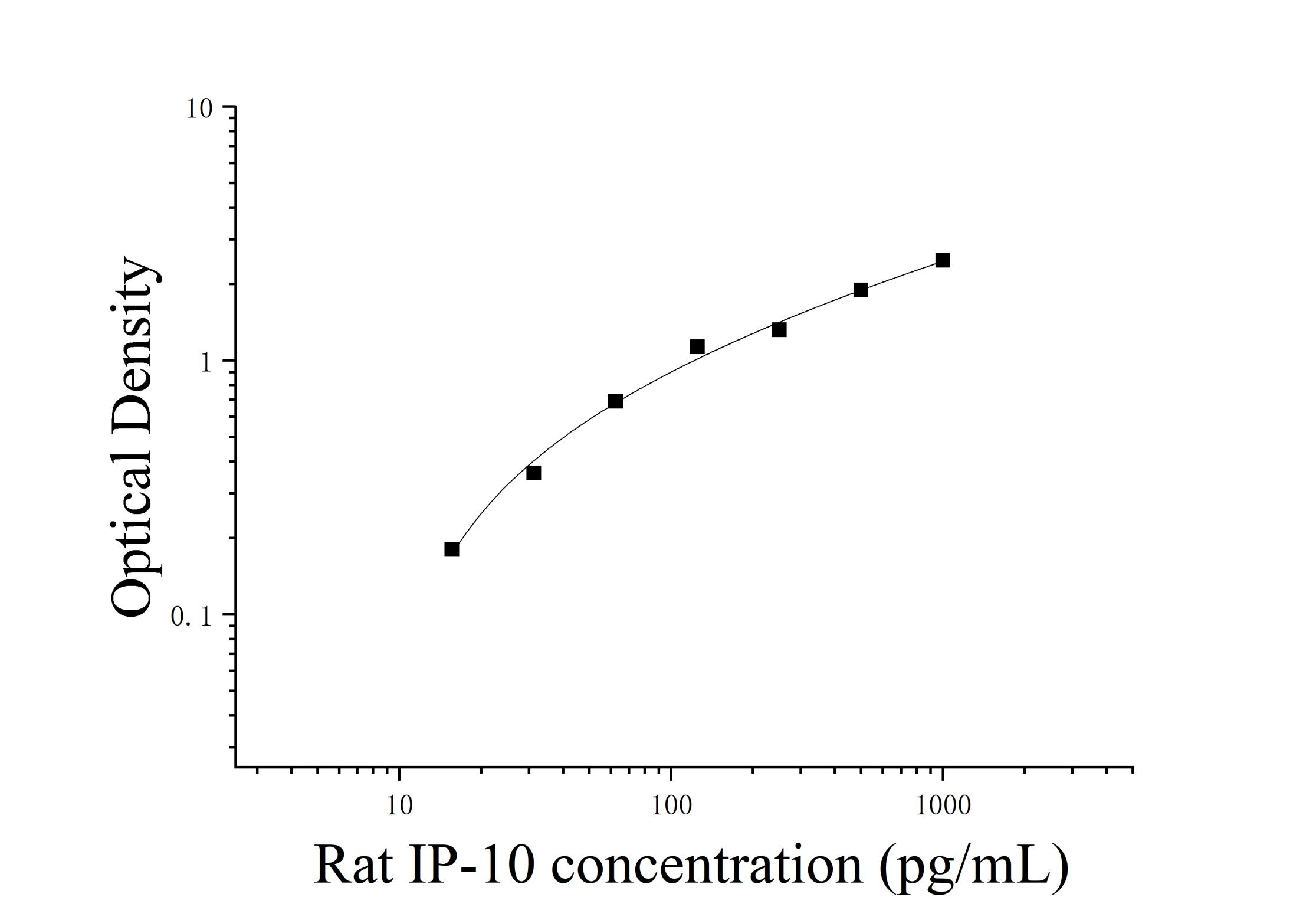
Cell Culture Supernatant: Centrifuge at 1000×g for 20 minutes. Collect the supernatant for analysis or store at -20°C or -80°C, but avoid repeated freeze-thaw cycles. Other biological fluids: Centrifuge at 1000xg for 20 minutes, remove the supernatant, and assay. Pre-Assay Preparation: 1. Remove the test kit from the refrigerator 10 minutes in advance and equilibrate to room temperature. 2. Prepare the standard gradient working solution: Add 1 mL of universal diluent to the lyophilized standard, let stand for 15 minutes to completely dissolve, then gently mix (concentration 1000 pg/mL). Then dilute to the following concentrations: 1000 pg/mL, 500 pg/mL, 250 pg/mL, 125 pg/mL, 62.5 pg/mL, 31.25 pg/mL, 15.625 pg/mL, and 0 pg/mL. Serial dilution method: Take seven EP tubes and add 500uL of universal diluent to each. Pipette 500uL of the 1000pg/mL standard working solution into the first EP tube and mix thoroughly to make a 500pg/mL standard working solution. Repeat this procedure for subsequent tubes. The last tube serves as a blank well; there is no need to pipette liquid from the penultimate tube. See the figure below for details. 3. Preparation of biotinylated detection antibody working solution: 15 minutes before use, centrifuge the concentrated biotinylated antibody at 1000×g for 1 minute. Dilute 100× concentrated biotinylated antibody to a 1× working concentration with universal diluent (e.g., 10 μL concentrate + 990 μL universal diluent). Prepare the solution before use. 4. Preparation of Enzyme Conjugate Working Solution: 15 minutes before use, centrifuge 100 μL of concentrated enzyme conjugate at 1000 × g for 1 minute. Dilute the 100 μL concentrated HRP enzyme conjugate with universal diluent to a 1 μL working concentration (e.g., 10 μL concentrate + 990 μL universal diluent). Prepare freshly for use. 5. Preparation of 1 μL Wash Solution: Dissolve 10 mL of 20 μL Wash Solution in 190 mL of distilled water. (Crystallization may occur in the concentrated wash solution after removal from the refrigerator. This is normal. Allow to stand at room temperature until the crystals have completely dissolved before preparation.) Procedure: 1. After equilibration at room temperature for 10 minutes, remove the desired strips from the aluminum foil bag. Seal the remaining strips in a ziplock bag and return them to 4°C. 2. Sample Addition: Add 100 μL of sample or standard of varying concentrations to the appropriate wells. Add 100 μL of universal diluent to the blank wells. Cover with film and incubate at 37°C for 60 minutes. (Recommendation: Dilute the test samples at least 1-fold with universal diluent before adding them to the plate. This will minimize matrix effects on the test results. When calculating sample concentrations, multiply by the dilution factor. It is recommended to run replicates for all test samples and standards.) 3. Biotinylated Antibody Addition: Remove the plate, discard the liquid, and do not wash. Add 100 μL of biotinylated antibody working solution directly to each well. Cover with film and incubate at 37°C for 60 minutes. 4. Wash: Discard the liquid and add 300 μL of 1x wash buffer to each well. Let stand for 1 minute, shake off the wash buffer, and pat dry on absorbent paper. Repeat this process three times (a microplate washer can also be used). 5. Add enzyme conjugate working solution: Add 100 μL of enzyme conjugate working solution to each well, cover with a film sealer, and incubate at 37°C for 30 minutes. 6. Wash: Discard the liquid and wash the plate five times according to the washing method in step 4. 7. Add substrate: Add 90 μL of substrate (TMB) to each well, cover with a film sealer, and incubate at 37°C in the dark for 15 minutes. 8. Add stop solution: Remove the ELISA plate and add 50 μL of stop solution directly to each well. Immediately measure the OD value of each well at 450 nm. Calculation of Experimental Results: Interpretation of Results: 1. Calculate the average OD value of the standard and sample replicates and subtract the OD value of the blank well as the correction value. Plot the standard curve of the four-parameter logistic function on double-logarithmic graph paper, with concentration as the horizontal axis and OD value as the vertical axis. 2. If the sample OD value is higher than the upper limit of the standard curve, dilute the sample appropriately and retest. Multiply the sample concentration by the corresponding dilution factor.  Note: Note:The standard curve data provided is for reference only. Sample content should be calculated based on the standard curve drawn for the same experiment. Kit Parameters:1. Recovery: Rat CXCL10 was spiked into selected healthy rat serum, plasma, and cell culture supernatant at three different concentrations, and the recovery was calculated. The recovery ranged from 84-108%, with an average recovery of 98%. 2. Sensitivity: 4.73 pg/mL. 3. Linearity: High concentrations of rat CXCL10 were added to four samples of healthy rat serum, plasma, and cell culture supernatant, and diluted within the kinetic range of the standard curve to assess linearity. | Dilution ratio | Recovery rate (%) | Serum | Plasma | Cell culture supernatant | | 1:2 | Range | 84-95 | 88-96 | 90-110 | | Average recovery rate | style="width: 18.1755%;">Range | 89-103 | 87-108 | 105-115 | | Average recovery rate | 94 | 98 | 108 |
 |
| Background | Interferon-induced protein 10 (IP-10/CXCL10), also known as interferon-γ-induced protein 10 (IP-10) or small cytokine-induced B10, is an 8.7 kDa protein encoded by the CXCL10 gene. CXCL10 is secreted by several cell types in response to IFN-γ, including monocytes, endothelial cells, and fibroblasts. CXCL10 is believed to have multiple effects, including chemoattraction of monocytes/macrophages, T cells, NK cells, and dendritic cells, promotion of T cell adhesion to endothelial cells, anti-tumor activity, and inhibition of bone marrow colony formation and angiogenesis. This chemokine exerts its effects by binding to the cell surface chemokine receptor CXCR3. |





 Note:The standard curve data provided is for reference only. Sample content should be calculated based on the standard curve drawn for the same experiment.
Note:The standard curve data provided is for reference only. Sample content should be calculated based on the standard curve drawn for the same experiment.