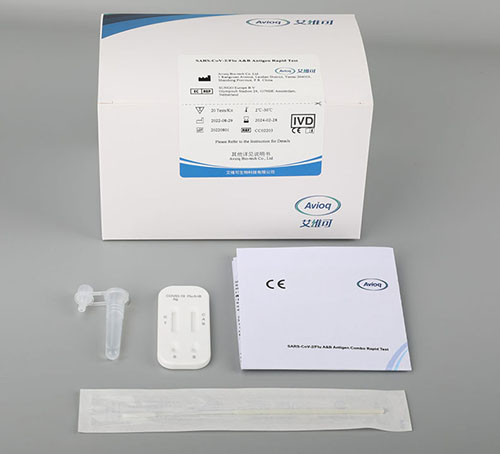
Intended Use
The test is a rapid colloidal gold immunochromatographic assay for the in vitro qualitative detection of the novel coronavirus(SARS-CoV-2), the influenza A virus (FluA) and the influenza B (FluB) antigen in human nasopharyngeal swab, oropharyngeal swab, nasal swab, and saliva samples from individuals suspected of respiratory viral infection.
Product Description
Rapid Influenza A and influenza B Antigen Combo Test Kit cassette Card
| Specification |
20tests/kit |
| Test Method |
Colloidal Gold |
| Power source |
Manual |
| Shelf life |
See manufacture date on label |
| Qualification |
EU CE, ISO13485:2016 |
| Function |
Home test/For professional use |
| Service |
OEM services are avaliable |

Test Procedure
1.Before testing, equilibrate the sample extract ion buffer to room temperature. Insert the test extraction tube into the work station.Make sure that the tube is standing firm and reaches the bottom of the workstation
2.Tear off the aluminum foil paper of t he extraction tube that contains sample extraction buffer. Insert the collected swab samples into the extraction tube.
3. Roll the swab at least 6 times while pressing the head against the bottom and side of the extracti on tube.
4. Leave the swab in the extraction tube for 1 minute.
5. Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab. The extracted solution will be used as test sample. Cover the extraction tube with the dropper tip tightly.
6.Allow the detection card and test sample solution to equilibrate to room temperature. Take out the detection card from the package just before testing and place it on a clean and level surface.
7. Reverse the sample extraction tube , with the dropper downwards. A dd 4 drops of test sample solution ( by squeezing the tube into each of the two sample wells on the detection card.
8. Wait for the colored band(s) to appear. The result s should be read in 15 minutes. Do not interpret the result s longer than 15 minutes.
FAQ
1.Delivery date
A:leading time of sample order is about 3-5 wordays.
2.Shipment
A:We usually ship by DHL, UPS, FedEx. It usually takes 3-5 days to arrive.Airline and sea shipping is also optional.
3.If you accept OEM?
A:yes,OEM is avaliable
4.Will you help me register the products in my country?
A:we would like offer technical documents and support to help you to register the products until the registration is finished.
5.Do you have MOQ?
Low MOQ, 1pc for sample checking is available
bio-equip.cn