Home > News > Unlocking the Potential of CD20-Targeted CAR-T Therapy: Advancing Treatment for B-Cell Non-Hodgkin Lymphoma
Unlocking the Potential of CD20-Targeted CAR-T Therapy: Advancing Treatment for B-Cell Non-Hodgkin Lymphoma
Concept
CD20, a B lymphocyte-restricted differentiation antigen encoded by the MS4A1 gene, is a 35 kDa tetraspanning membrane protein. It exhibits strict lineage specificity, being predominantly expressed on pre-B cells and mature B lymphocytes while absent from hematopoietic stem cells, pro-B cells, normal plasma cells, and other non-target tissues. This unique expression profile, coupled with its role in regulating B-cell activation and proliferation via calcium channel modulation, positions CD20 as a prime therapeutic target for B-cell malignancies.Research Frontiers
1. Addressing Limitations of CD19 CAR-T Therapy: Despite the notable efficacy of CD19 CAR-T in B-cell malignancies, clinical data indicate a complete response rate of only around 50% in non-Hodgkin lymphoma patients, with approximately 20% developing primary resistance. Antigen escape—where malignant B cells downregulate or lose CD19 expression to evade immune detection—stands as a key failure mechanism. CD20, with its distinct expression timeline during B-cell development and independent downregulation pathways compared to CD19, offers a promising alternative to overcome target loss-related treatment failures.
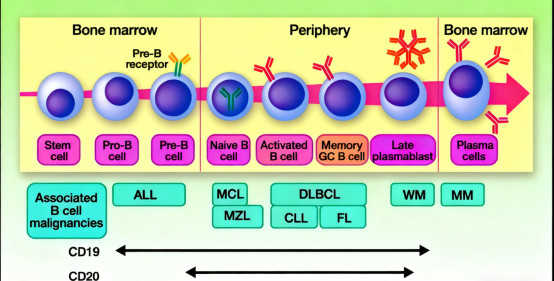
2. Biological Underpinnings Supporting Therapeutic Application: From a developmental biology standpoint, CD20 expression spans multiple B-cell stages, from pre-B cells to mature B cells, but is absent in terminally differentiated plasma cells. This profile ensures coverage of most B-cell malignancies while sparing critical immune cell populations like plasma cells, minimizing off-target effects. Additionally, CD20’s stable membrane localization and consistent conformational features facilitate sustained immune synapse formation—an advantage over targets prone to internalization or shedding—making it an ideal recognition motif for CAR-T cells.
3. Technical Challenges and Innovation Opportunities: Developing CD20 CAR-T therapy requires addressing key technical hurdles, including optimizing CAR molecule design for high affinity and specificity to CD20, balancing therapeutic potency with safety to avoid excessive depletion of normal B cells (which could lead to immunodeficiency), and accounting for CD20 expression heterogeneity in certain B-cell lymphomas. However, these challenges open avenues for innovation: sequential or combinatorial use of CD20 and CD19 CAR-T to prevent antigen escape; exploration of synergies with existing CD20-targeted agents; and development of CAR constructs targeting distinct CD20 epitopes to enhance efficacy.
4. Clinical Positioning: CD20-targeted therapy holds multi-faceted clinical value in current treatment paradigms. It serves as a critical alternative for patients who fail or are ineligible for CD19 CAR-T therapy. In combination strategies, CD20-targeted agents complement CAR-T therapy through synergistic mechanisms. Furthermore, patient stratification based on CD20 expression levels enables more precise personalized treatment. As insights into resistance mechanisms deepen, the clinical utility of CD20-targeted therapy continues to expand.
5. Future Directions: The future of CD20-targeted therapy lies in multiple innovative avenues. Novel CAR architectures aim to improve persistence and safety through modulated co-stimulatory signals or integrated safety switches. Optimized combination strategies include synergistic pairing with immune checkpoint inhibitors and small-molecule targeted drugs. Expanded indications involve exploring applications in a broader spectrum of B-cell malignancies. Additionally, the development of predictive biomarkers and resistance-reversal strategies remains a key research focus, driving CD20-targeted therapy forward in the era of precision medicine.
Research Significance
CD20’s unique biological characteristics and expression pattern address a critical unmet need in B-cell malignancy treatment—overcoming antigen escape in CD19 CAR-T therapy. By providing a viable alternative target, CD20-targeted strategies enhance treatment efficacy, expand therapeutic options for refractory patients, and pave the way for more durable clinical responses. Moreover, advancements in CD20-targeted therapy contribute to the evolution of precision medicine in hematological oncology, enabling tailored treatments that balance efficacy and safety.
Related Mechanisms, Research Methods, and Product Applications
Mechanisms
CD20 regulates B-cell activation and proliferation through calcium channel modulation, and its stable membrane expression supports sustained engagement with CAR-T cells, triggering targeted cytotoxicity against malignant B cells. The independent downregulation pathways of CD20 and CD19 allow for combinatorial targeting to prevent antigen escape.
Research Methods
Key research methods in CD20-targeted therapy include:
* Immunohistochemistry (IHC) for CD20 expression detection and quantification in tissue samples.
* Flow cytometry for analyzing B-cell populations and CD20 expression levels in peripheral blood or bone marrow.
* CAR-T cell engineering and functional validation (e.g., cytotoxicity assays, in vivo tumor models) to optimize CD20-specific CAR constructs.
* Clinical trials evaluating the efficacy, safety, and pharmacodynamics of CD20 CAR-T therapy and combination regimens.
Product Applications
ANT BIO PTE. LTD.’s CD20 antibodies play a pivotal role in advancing CD20-targeted research and clinical translation:
* B-Cell Lymphoma Diagnosis & Subtyping: Enables accurate diagnosis and differentiation of diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, and other B-cell malignancies through specific CD20 detection.
* B-Cell Quantification & Analysis: Facilitates qualitative and quantitative assessment of B cells in normal lymphoid tissues, inflammatory tissues, and tumor microenvironments, supporting research on disease pathogenesis.
* Immunotherapy Efficacy Evaluation: Serves as a companion diagnostic tool for CD20-targeted therapies (e.g., rituximab, CD20 CAR-T), aiding in patient screening, treatment response monitoring, and prognosis assessment.
* Autoimmune Disease Research: Supports the detection and analysis of B-cell infiltration in autoimmune conditions such as rheumatoid arthritis and multiple sclerosis.
A standout product is the "S-RMab® CD20 Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2158) from ANT BIO PTE. LTD.’s STARTER brand. Developed using the proprietary S-RMab® recombinant rabbit monoclonal antibody platform and validated for IHC and other techniques, it offers high specificity with clear membrane localization (ensuring reliable B-cell identification in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnosis and translational research.
Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community by providing high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms (including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid mouse monoclonal antibody, and recombinant protein expression systems) and rigorous quality control (compliant with EU 98/79/EC, ISO9001, and ISO13485 certifications), we strive to support breakthroughs in research and clinical translation, ultimately advancing human health through scientific innovation.
Related Product List
| Catalog No. | Product Name | Host |
| S0B0547 | Invivo Recombinant anti-mouse CD20 antibody | Mouse |
| S0B5896 | Alexa Fluor® 700 Mouse Anti-Human CD20 Antibody (S-R449) | Mouse |
| S0B8283 | Pacific Blue Mouse Anti-Mouse CD20 Antibody (18B12) | Mouse |
| S0B1511 | Alexa Fluor® 488 Mouse Anti-Mouse CD20 Antibody (18B12) | Mouse |
| S0B5440 | Pacific Blue Mouse Anti-Human CD20 Antibody (S-R449) | Mouse |
| S0B5427 | APC-Cy7 Mouse Anti-Human CD20 Antibody (S-R449) | Mouse |
| S0B2158 | S-RMab® CD20 Recombinant Rabbit Monoclonal Antibody (SDT-R133) | Rabbit |
AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Unlocking the Potential of CD20-Targeted CAR-T Therapy: Advancing Treatment for 1/30/2026
- MetP Pharma’s Neural Targeting Drug Delivery Technology Sets a New Benchmark for 1/30/2026
- CD182 Antibodies: Deciphering CXCR2’s Multifaceted Roles in the Tumor Microenvir 1/29/2026
- Lifeasible Launches Enhanced Bulk Segregant Analysis Service to Boost Plant Bree 1/29/2026
- Eyestem Receives CDSCO Approval to Initiate Phase 2 Randomized, Controlled Human 1/28/2026
- CD172a Antibodies: A Cornerstone Tool for Immune Regulation Research 1/27/2026
- CD170 Antibodies: Blocking Myeloid-Derived Suppressor Cell-Mediated Tumor Immune 1/26/2026
- CD163 Antibodies: Targeted Delivery Systems for Promoting Vascular Regeneration 1/25/2026
- CD14 Antibodies: Deciphering Dual Regulatory Mechanisms in Inflammasome Activati 1/24/2026


