Home > News > How Can Immunohistochemistry Kits Enhance Experimental Efficiency and Result Reliability?
How Can Immunohistochemistry Kits Enhance Experimental Efficiency and Result Reliability?
- Modern IHC kits are designed to provide researchers with a standardized and streamlined protocol by systematically integrating key reagents required for the experiment.
Recent Advances
1. How does the design philosophy of IHC kits optimize the experimental workflow?
Modern IHC kits are designed to provide researchers with a standardized and streamlined protocol by systematically integrating key reagents required for the experiment. These kits typically include all necessary components for the entire process, from antigen retrieval to chromogenic detection, such as endogenous enzyme blockers, serum blocking buffers, enzyme-labeled secondary antibodies, and chromogenic substrates. Through carefully formulated reagent compositions and optimized reaction conditions, these kits effectively reduce operational complexity, minimize human error, and ensure the stability and reproducibility of results.
In terms of workflow optimization, the kits feature ready-to-use reagents, eliminating tedious preparation and concentration optimization steps. This design not only significantly shortens pre-experiment preparation time but also reduces the risk of failure due to improper reagent preparation. Notably, the optimized incubation times allow the entire staining process to be completed quickly, greatly improving experimental efficiency while maintaining reaction specificity and sensitivity.
2. How do the kits ensure the specificity and sensitivity of detection results?
IHC kits employ multiple technical strategies to ensure detection specificity and sensitivity. To enhance specificity, the kits incorporate efficient endogenous enzyme blocking systems that thoroughly eliminate interference from endogenous peroxidases or alkaline phosphatases in tissues. Simultaneously, optimized blocking buffer formulations effectively block non-specific binding sites, significantly reducing background staining. These designs ensure the specificity of the final chromogenic reaction, providing accurate and reliable localization of the target antigen.
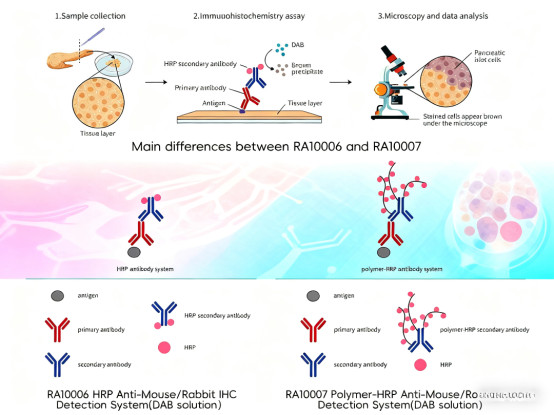
For sensitivity improvement, modern kits often use polymer labeling technology instead of the traditional streptavidin-biotin system. This technology conjugates multiple enzyme molecules to a polymer backbone attached to the secondary antibody, creating a highly efficient signal amplification system. Compared to traditional enzyme-labeled secondary antibodies, the polymer system not only significantly improves detection sensitivity but also avoids background interference caused by endogenous biotin in tissues. Furthermore, rigorously quality-controlled chromogenic substrates ensure stable and reproducible signal output, enabling the detection of low-abundance antigens.
3. How does the application range of the kits meet diverse experimental needs?
High-quality IHC kits should have a broad application range to meet the needs of various research scenarios. Regarding sample types, the kits should be suitable for multiple sample formats, including formalin-fixed paraffin-embedded (FFPE) tissues, frozen tissue sections, and cell smears. This wide applicability allows researchers to compare antigen expression across different sample sources under uniform experimental conditions, ensuring result comparability.
Regarding the range of detectable antigens, the kits should be compatible with targets of diverse nature. Whether detecting cell membrane proteins, cytoplasmic antigens, or nuclear antigens, the kit should provide reliable detection performance. Additionally, the kits should exhibit good antibody compatibility, working effectively with primary antibodies from different species, offering greater flexibility in experimental design. This comprehensive applicability enables researchers to complete multiple projects using the same kit, significantly improving experimental efficiency and reducing research costs.
4. How does standardized operation ensure experimental result reproducibility?
IHC kits ensure result reproducibility through standardized operating procedures. The kits provide detailed, standardized protocols covering critical parameters such as reagent application sequence, incubation times, and temperature control. These standardized procedures not only help maintain operational consistency within a laboratory but also facilitate comparison and communication of results between different laboratories.
Regarding quality control, high-quality kits provide clear quality control standards and recommendations for appropriate control settings. Through these quality control measures, researchers can promptly identify and troubleshoot issues during the experiment, ensuring result reliability. Furthermore, standardized interpretation criteria help reduce the impact of subjective factors on result analysis, enhancing the scientific rigor and credibility of the experimental data.
5. How does a comprehensive quality control system guarantee kit performance?
A robust quality control system is crucial for ensuring the performance of IHC kits. During the development phase, the kits undergo rigorous methodological validation, including sensitivity testing, specificity assessment, and inter-batch consistency checks. These validations ensure stable kit performance under various experimental conditions.
During manufacturing, strict raw material screening and quality control form the foundation of kit quality. Each batch of reagents undergoes comprehensive performance testing to ensure it meets established quality standards. Additionally, thorough stability studies provide a scientific basis for kit storage and use, ensuring no significant performance degradation within the shelf life.
By establishing a complete technical support system, kit suppliers can promptly address user issues and collect feedback for continuous product improvement. This all-encompassing quality assurance system ensures that IHC kits deliver reliable and accurate results under various experimental conditions, providing strong technical support for scientific research.
6. Which companies provide IHC kits?
Hangzhou Start Biological Technology Co., Ltd. independently developed the "Anti-Rabbit/Mouse HRP-DAB IHC Detection Kit (2-Step)" (Product Name: Anti-Rabbit and Mouse HRP-DAB IHC detection kit (2-step), Catalog Number: S0C2011). This is an IHC detection system characterized by high sensitivity, low background interference, and excellent chromogenic stability. The product utilizes an innovative two-step detection technology that combines enzyme-labeled polymer technology with high-purity DAB chromogenic substrate, demonstrating outstanding performance in IHC detection of various samples, including paraffin sections and frozen sections.
Core Product Advantages:
* High Sensitivity & Low Background: Utilizing high-specific-activity HRP labeling and an optimized blocking system, this product effectively amplifies specific signals while significantly reducing non-specific background. Its innovative two-step design simplifies the operational workflow, reduces experimental error, and ensures sharp, clear staining results.
* Excellent Chromogenic Stability & Batch Consistency: Core components undergo strict quality control verification. The DAB substrate offers superior light stability and chromogenic reproducibility, allowing stained results to be preserved long-term. Minimal intra-batch and inter-batch variation provides stable and reliable assurance for both research and clinical diagnostics.
Suitable for Key Application Scenarios:
This product is an ideal tool for the following research areas:
* Clinical Pathological Diagnosis: Suitable for routine IHC detection in hospital pathology departments, used for tumor diagnosis, typing, and detection of prognostic markers (e.g., ER, PR, HER2, Ki-67).
* Basic Research & Translational Medicine: Used for the localization and semi-quantitative analysis of target proteins in FFPE tissues and frozen sections, widely applicable in oncology, neuroscience, metabolism, and other research fields.
* Drug Discovery & Biomarker Validation: Used for the localization of drug targets and the detection/validation of pharmacodynamic biomarkers in preclinical research.
* Automated Staining Platforms: Its stable performance makes it compatible with mainstream automated IHC stainers, meeting high-throughput detection needs.
Professional Technical Support:
We provide detailed product technical documentation, including standard operating procedures, optimization suggestions, troubleshooting guides, and chromogenic result comparisons, fully assisting customers in achieving optimal staining outcomes.
Hangzhou Start Biological Technology Co., Ltd. is committed to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. To learn more about the "Anti-Rabbit/Mouse HRP-DAB IHC Detection Kit (2-Step)" (Catalog Number S0C2011) or to request a sample for testing, please feel free to contact us.
| Catalog Number | Product Name | Product Parameters |
| S0C2031 | Anti-Rabbit HRP-DAB IHC detection kit | Host : Goat Conjugation : HRP |
| S0C1001 | Anti-Rabbit and Mouse HRP&DAB IHC detection kit | Host : Goat Conjugation : HRP |
| S0C2011 | Anti-Rabbit and Mouse HRP-DAB IHC detection kit (2-step) | Host : Goat Conjugation : HRP |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026
- How to Understand the Core Structure and Immune Functions of Ig Antibodies? 1/20/2026
- CD163 Antibody: How to Promote Vascular Regeneration and Homeostasis Recovery af 1/19/2026


