NTENDED USE
The SARS-COV-2 Antigen Rapid Test Device is a rapid visual immunoassay for the qualitative, presumptive detection of COVID-19 antigens form throat swabs and nasopharyngeal swab specimens.
It is intended to be used by professionals as a test and provides a preliminary test result to aid in the diagnosis of infection with novel Coronavirus.
Any interpretation or use of this preliminary test result must also rely on other clinical findings as well as on the professional judgment of health care providers. Alternative test method(s) should be considered to confirm the test result obtained by this test.
INTRODUCTION
The novel coronaviruses (SARS-CoV-2) belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
KIT COMPONENTS
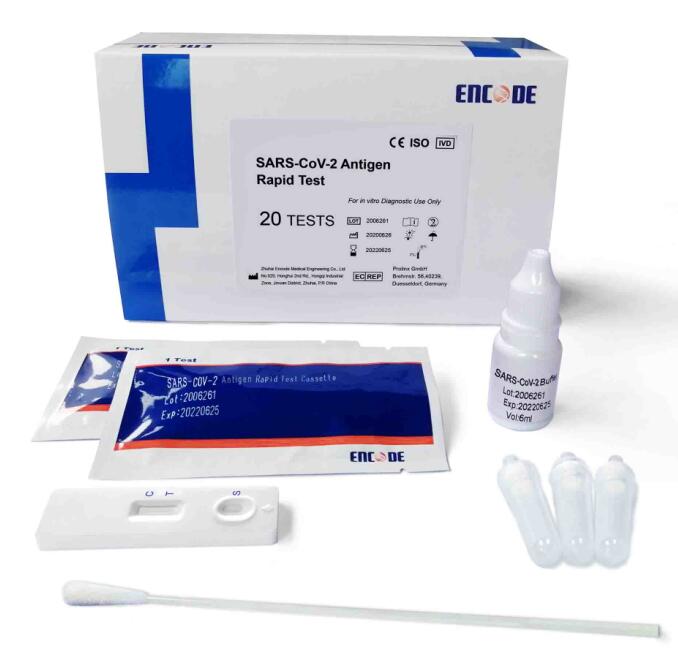
|
Individually packed Test Devices
|
Each test contains colored conjugates and reactive reagents precoated at the corresponding regions
|
|
Extraction solution
|
For specimens extraction.
|
|
Extraction tubes
|
For specimen preparation
|
|
Sterile nasal swabs
|
For specimen collection
|
|
Package insert
|
For operating instructions
|
PROCEDURE
Bring tests, specimens, and/or controls to room temperature (15-30°C) before use.
- Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. For best results, the assay should be performed within one hour.
- Gently mix Extraction reagent solution. Add 6 drops(about 200ul) of the Extraction Solution into the Extraction tube.
- Place the patient swab specimen into the Extraction Tube. Roll the swab at least 10 times while pressing the swab against the bottom and side of the Extraction Tube. Roll the swab head against the inside of the Extraction Tube as you remove it. Try to release as much liquid as possible. Dispose of the used swab in accordance with your biohazard waste disposal protocol.
- Put on the tube tip, then add 3 drops (about 120ul) of extracted sample into the sample well. Do not handle or move the Test Device until the test is complete and ready for reading.
- As the test begins to work, color will migrate across the membrane. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
PERFORMANCE CHARACTERISTICS
Relative Sensitivity: 86.67% (78.07%~95.27%)
Relative Specificity: 100% (99.8%~100%)
Overall Agreement: 95.09% (91.78%~98.41%)
*95% Confidence Interval
bio-equip.cn