Home > News > AMSBIO: New Tissue Microarrays Conform with FDA Standards
AMSBIO: New Tissue Microarrays Conform with FDA Standards
AMSBIO offers an unmatched portfolio of over 2000 tissue microarrays (TMAs), one of the best sample formats for rapid detection of cellular localization of RNA and protein expression.
Recently AMSBIO introduced US Food & Drug Administration (FDA) standard TMAs for human, mouse and rat to its portfolio. Designed in close conformance with FDA guidelines, this range meets the requirements for tissue cross-reactivity studies required for therapeutic and diagnostic antibody and antibody-like molecules validation. Each array slide features the FDA recommended list of normal tissue types in triplicate, allowing the identification of off-target binding and on-target binding that has not been previously identified.
Tissue microarray slides offer a rapid and cost-effective direct method for the validation of biological targets in multiple tissue samples. Parallel analysis of multiple specimens reduces operating times, conserves reagents and facilitates high-throughput molecular analysis of tissues.
Recently AMSBIO introduced US Food & Drug Administration (FDA) standard TMAs for human, mouse and rat to its portfolio. Designed in close conformance with FDA guidelines, this range meets the requirements for tissue cross-reactivity studies required for therapeutic and diagnostic antibody and antibody-like molecules validation. Each array slide features the FDA recommended list of normal tissue types in triplicate, allowing the identification of off-target binding and on-target binding that has not been previously identified.
Tissue microarray slides offer a rapid and cost-effective direct method for the validation of biological targets in multiple tissue samples. Parallel analysis of multiple specimens reduces operating times, conserves reagents and facilitates high-throughput molecular analysis of tissues.
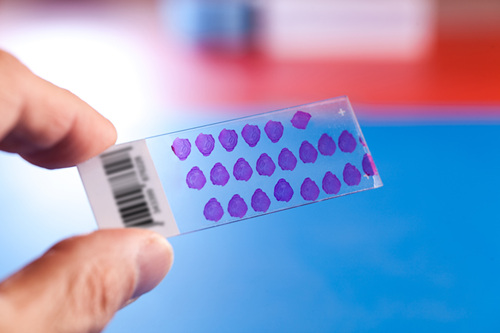
The new FDA standard TMAs form part of AMSBIO’s broad collection of normal and tumor TMAs available in FFPE and frozen formats. Produced using high-quality tissue samples selected by board-certified pathologists, TMAs are a remarkable tool with a range of potential applications in basic research, prognostic oncology and drug discovery.
For further information on high quality tissue microarrays please visit http://www.amsbio.com/tissue-samples-frozen-ffpe-blocks-sections-arrays.aspx or contact AMSBIO on +44-1235-828200 / +1-617-945-5033 / info@amsbio.com.
Founded in 1987, AMS Biotechnology (AMSBIO) is recognized today as a leading transatlantic company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products and services for research and development in the medical, nutrition, cosmetics and energy industries. AMSBIO has in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion and proliferation. This expertise in cell culture and the ECM allows AMSBIO to partner with clients in tailoring cell systems to enhance organoid and spheroid screening outcomes using a variety of 3D culture systems, including organ-on-a-chip microfluidics.
For drug discovery research, AMSBIO offers assays, recombinant proteins and cell lines. Drawing upon a huge and comprehensive biorepository, AMSBIO is widely recognised as a leading provider of high-quality tissue specimens (including custom procurement) from both human and animal tissues. The company provides unique clinical grade products for stem cell and cell therapy applications these include high quality solutions for viral delivery (lentivirus, adenovirus and adeno-associated virus) in addition to GMP cryopreservation technology. For further information please visit www.amsbio.com
Worldwide HQ
AMS Biotechnology (AMSBIO)
For further information on high quality tissue microarrays please visit http://www.amsbio.com/tissue-samples-frozen-ffpe-blocks-sections-arrays.aspx or contact AMSBIO on +44-1235-828200 / +1-617-945-5033 / info@amsbio.com.
Founded in 1987, AMS Biotechnology (AMSBIO) is recognized today as a leading transatlantic company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products and services for research and development in the medical, nutrition, cosmetics and energy industries. AMSBIO has in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion and proliferation. This expertise in cell culture and the ECM allows AMSBIO to partner with clients in tailoring cell systems to enhance organoid and spheroid screening outcomes using a variety of 3D culture systems, including organ-on-a-chip microfluidics.
For drug discovery research, AMSBIO offers assays, recombinant proteins and cell lines. Drawing upon a huge and comprehensive biorepository, AMSBIO is widely recognised as a leading provider of high-quality tissue specimens (including custom procurement) from both human and animal tissues. The company provides unique clinical grade products for stem cell and cell therapy applications these include high quality solutions for viral delivery (lentivirus, adenovirus and adeno-associated virus) in addition to GMP cryopreservation technology. For further information please visit www.amsbio.com
Worldwide HQ
AMS Biotechnology (AMSBIO)
184 Milton Park
Abingdon
Abingdon
Oxon OX14 4SE
North American HQ
AMSBIO LLC
1035 Cambridge Street
Cambridge
MA 02141
USA
Tel: +1.617.945.5033
Tel: +1.800.987.0985 (toll free)
Email: info@amsbio.com
Web www.amsbio.com
1035 Cambridge Street
Cambridge
MA 02141
USA
Tel: +1.617.945.5033
Tel: +1.800.987.0985 (toll free)
Email: info@amsbio.com
Web www.amsbio.com
Related News
- Low Binding DNA Consumables for NGS and Molecular Biology 1/30/2026
- Creative BioMart Launches GenePowerTM Controlled Randomization to Support Precis 1/29/2026
- Biotech Fluidics: Instrument Optimized Vacuum Degassers. 1/28/2026
- High Speed Framing Cameras for Fusion Research 1/21/2026
- Tri-coded Storage Tube Optimized for Maximum Sample Recovery 1/16/2026
- Optimised UV Lenses for Forensic Investigation 1/16/2026
- Large Format Aerial Surveillance Lens 1/7/2026
- Breathing New Life into Older High-speed Cameras 1/7/2026
- New Handheld Decapper Boosts Speed and Consistency of 48-Format Tube Handling 1/6/2026
- Celleo Biotech Uses INTEGRA's PIPETBOY GENIUS to Improve Reproducibility and Eff 1/5/2026


