Home > News > AMSBIO: High Throughput PARP in vivo Pharmacodynamic Assay
AMSBIO: High Throughput PARP in vivo Pharmacodynamic Assay
AMSBIO has introduced a unique High Throughput PARP in vivo Pharmacodynamic Assay that can be used to monitor the efficacy of poly-ADP-ribose polymerase (PARP) inhibitors on PAR formation in vivo; and to measure drug action on PARP in both in vivo and in vitro settings
In recent news, AstraZeneca reported upon how their ovarian cancer drug Lynparza™ (olaparib) is the first PARP inhibitor to be granted approval by the European Commission and the US Food and Drug Administration (FDA)**. This has led to a surge of interest in this class of drugs, which can exploit tumour DNA repair pathway deficiencies to preferentially kill cancer cells.
Dr William Hadlington-Booth of AMSBIO commented "Our High Throughput PARP in vivo Pharmacodynamic Assay is helping facilitate the development of PARP and PARG targeted therapeutics by many pharmaceutical companies and clinical researchers worldwide. Research work has already shown its utility in measuring PAR levels in peripheral blood mononuclear cells, tissue culture cells, and tumor lysates from different tissues, organs and xenografts: it can be used to monitor the efficacy of PARP inhibitors on PAR formation in vivo, as well as to verify observations of enhanced cancer cell cytotoxicity arising from PARP inhibitor/anticancer drug combination therapy".
In recent news, AstraZeneca reported upon how their ovarian cancer drug Lynparza™ (olaparib) is the first PARP inhibitor to be granted approval by the European Commission and the US Food and Drug Administration (FDA)**. This has led to a surge of interest in this class of drugs, which can exploit tumour DNA repair pathway deficiencies to preferentially kill cancer cells.
Dr William Hadlington-Booth of AMSBIO commented "Our High Throughput PARP in vivo Pharmacodynamic Assay is helping facilitate the development of PARP and PARG targeted therapeutics by many pharmaceutical companies and clinical researchers worldwide. Research work has already shown its utility in measuring PAR levels in peripheral blood mononuclear cells, tissue culture cells, and tumor lysates from different tissues, organs and xenografts: it can be used to monitor the efficacy of PARP inhibitors on PAR formation in vivo, as well as to verify observations of enhanced cancer cell cytotoxicity arising from PARP inhibitor/anticancer drug combination therapy".
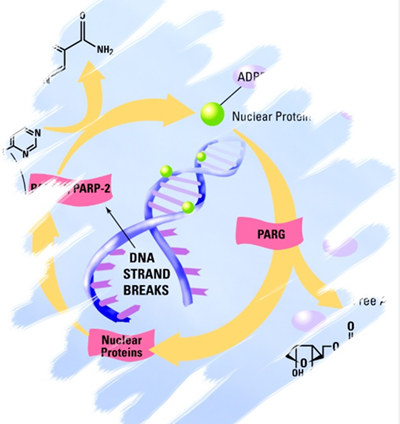
Validated to USP recommended guidelines and fully compatible with laboratory automation systems, this AMSBIO high throughput kit is the only PAR ELISA assay currently available.
Pharmacodynamic assays measure biomarkers that can quantify the molecular and functional effects produced by a drug on its intended target. Since pharmacodynamic biomarkers play important roles in deciphering disease processes and mechanisms of drug action, validated pharmacodynamic assays that accurately measure the effects of a drug on its target are becoming an essential component to clinical trials and the drug development paradigm.
For further information on the High Throughput PARP in vivo Pharmacodynamic Assay please visit www.amsbio.com/parp-in-vivo-pharmacodynamic-assay.aspx or contact AMSBIO now on +44-1235-828200 / +1-617-945-5033 or email info@amsbio.com.
Founded in 1987, AMS Biotechnology (AMSBIO) is recognized today as a leading company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products and services for research and development in the medical, nutrition, cosmetics and energy industries. AMSBIO is able to draw upon in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion and proliferation. Widely acknowledged as an expert in cell culture, AMSBIO partners with clients in tailoring cell systems to enhance organoid and spheroid type screening outcomes from a technological and cost-effective perspective. Custom services include stable cell line production, CRISPR/Cas9 Genome Editing, lentivirus and adenovirus production, large scale protein production and a comprehensive portfolio of antibody services.
Pharmacodynamic assays measure biomarkers that can quantify the molecular and functional effects produced by a drug on its intended target. Since pharmacodynamic biomarkers play important roles in deciphering disease processes and mechanisms of drug action, validated pharmacodynamic assays that accurately measure the effects of a drug on its target are becoming an essential component to clinical trials and the drug development paradigm.
For further information on the High Throughput PARP in vivo Pharmacodynamic Assay please visit www.amsbio.com/parp-in-vivo-pharmacodynamic-assay.aspx or contact AMSBIO now on +44-1235-828200 / +1-617-945-5033 or email info@amsbio.com.
Founded in 1987, AMS Biotechnology (AMSBIO) is recognized today as a leading company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products and services for research and development in the medical, nutrition, cosmetics and energy industries. AMSBIO is able to draw upon in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion and proliferation. Widely acknowledged as an expert in cell culture, AMSBIO partners with clients in tailoring cell systems to enhance organoid and spheroid type screening outcomes from a technological and cost-effective perspective. Custom services include stable cell line production, CRISPR/Cas9 Genome Editing, lentivirus and adenovirus production, large scale protein production and a comprehensive portfolio of antibody services.
---------------------
Worldwide HQ
AMS Biotechnology (AMSBIO)
184 Milton Park
Abingdon
Abingdon
Oxon OX14 4SE
North American HQ
1035 Cambridge Street
Cambridge
MA 02141
USA
Tel: +1.617.945.5033
Tel: +1.800.987.0985 (toll free)
Email: info@amsbio.com
Web www.amsbio.com
Related News
- Ultra Smooth Hyperbolic Mirrors 2/9/2026
- Low Binding DNA Consumables for NGS and Molecular Biology 1/30/2026
- Creative BioMart Launches GenePowerTM Controlled Randomization to Support Precis 1/29/2026
- Biotech Fluidics: Instrument Optimized Vacuum Degassers. 1/28/2026
- High Speed Framing Cameras for Fusion Research 1/21/2026
- Tri-coded Storage Tube Optimized for Maximum Sample Recovery 1/16/2026
- Optimised UV Lenses for Forensic Investigation 1/16/2026
- Large Format Aerial Surveillance Lens 1/7/2026
- Breathing New Life into Older High-speed Cameras 1/7/2026
- New Handheld Decapper Boosts Speed and Consistency of 48-Format Tube Handling 1/6/2026


