Home > News > Optimizing In Vitro T Cell Activation and Expansion: The Pivotal Role of CD3 Epsilon Antibodies
Optimizing In Vitro T Cell Activation and Expansion: The Pivotal Role of CD3 Epsilon Antibodies
- 1. Concept
Effective T cell activation is governed by a tightly regulated dual-signal system. CD3 epsilon (CD3ε), a key component of the T cell receptor (TCR) complex, serves as the molecular target for CD3 epsilon antibodies, which initiate the primary signal required for T cell activation. Complementing this, CD28 antibodies provide essential co-stimulatory signals, mimicking the natural interaction between T cells and antigen-presenting cells (APCs) in vivo. By covalently conjugating these specific monoclonal antibodies to 3.0μm polymer magnetic beads, a synergistic activation system is established—one that accurately replicates the microenvironmental conditions necessary for complete T cell activation and expansion in vitro. The size parameters of the magnetic beads and antibody conjugation technology have been meticulously optimized to ensure optimal stimulation signal density and spatial conformation within the culture system.
2. Research Frontiers
2.1 Molecular Basis of In Vitro T Cell Activation
The dual-signal mechanism underpins successful in vitro T cell activation: the primary signal is triggered by CD3 epsilon antibodies binding to the CD3ε chain of the TCR complex, while the co-stimulatory signal is delivered via CD28 antibody interaction with CD28 on T cells—mimicking the in vivo binding of CD28 to CD80/CD86 (B7-1/B7-2) on APCs. This combination of signals is indispensable for avoiding T cell anergy and inducing robust activation, proliferation, and functional maturation.
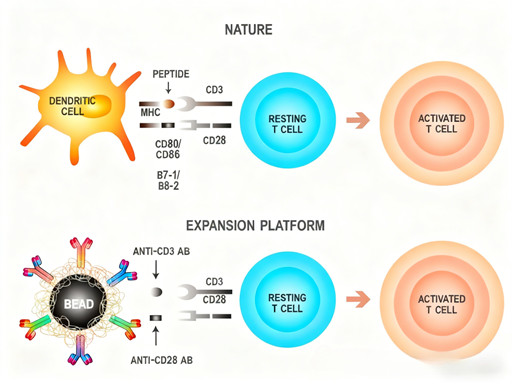
2.2 Technical Advantages of the CD3/CD28 Magnetic Bead Activation System
The CD3 epsilon antibody-based magnetic bead activation system offers multiple distinctive technical benefits:
Sustained and Stable Signaling: The precise ratio and spatial arrangement of CD3 epsilon and CD28 antibodies on the bead surface deliver continuous, consistent stimulation—avoiding the overactivation or signal attenuation commonly associated with soluble antibodies.
Optimal Immune Synapse Formation: The uniform size and surface properties of the magnetic beads create ideal contact interfaces with T cells, facilitating efficient immune synapse assembly, a critical step for effective signal transduction.
Easy Post-Activation Removal: Magnetic bead technology allows for straightforward separation of beads from activated T cells, minimizing potential interference with subsequent cell functions or downstream applications.
Flexible Stimulation Tuning: The system enables adjustable stimulation intensity and duration, catering to diverse experimental requirements—from short-term activation assays to long-term expansion protocols.
2.3 Establishing a Standard Operating Procedure (SOP) for T Cell Activation
Developing a standardized T cell activation protocol requires careful consideration of key parameters:
Initial Setup: A 1:1 cell-to-bead ratio is recommended for initiating activation, with cells cultured in a suitable medium optimized for T cell growth.
Environmental Control: Strict maintenance of 37°C temperature and appropriate CO₂ concentration (typically 5%) is essential to preserve cell viability and function.
Monitoring During Activation: Regular assessment of cell morphological changes (e.g., increased volume, irregular shape) and proliferation status (e.g., clone formation, cell count expansion) is required to track activation progress.
Long-Term Expansion: For extended culture, supplementation with cytokines such as interleukin-2 (IL-2) at optimal concentrations is necessary, along with scheduled medium changes and cell passaging to prevent overcrowding.
2.4 Evaluating T Cell Activation and Expansion Efficiency and Quality
A multi-level indicator system is employed to assess activation outcomes:
Morphological Assessment: Activated T cells exhibit characteristic features including enlarged cell volume, abundant cytoplasm, and increased granularity.
Phenotypic Analysis: Flow cytometry is used to detect expression levels of T cell activation markers (e.g., CD69, CD25, CD44), evaluating transduction efficiency and subset distribution (e.g., CD4⁺ vs. CD8⁺ T cells).
Functional Validation: Cytotoxicity assays (e.g., target cell killing assays) are critical to confirm the effector function of activated T cells, ensuring they retain the capacity to eliminate target cells.
Dynamic Monitoring: Real-time cell analysis (RTCA) technologies enable continuous tracking of T cell proliferation and killing activity, providing real-time feedback to optimize culture conditions.
2.5 Application Value in Cell Therapy
CD3 epsilon antibody-based T cell activation technology is a cornerstone of adoptive cell therapy, particularly in CAR-T cell development:
Efficient Gene Transduction: Optimized activation protocols enhance the efficiency of viral or non-viral gene delivery, with research data showing transduction efficiencies exceeding 70% in optimized systems.
Functional Effector Cells: Activated and expanded T cells (including CAR-T cells) demonstrate enhanced cytotoxic activity against target cells, a key attribute for therapeutic efficacy.
Standardization of Cell Products: The system ensures consistent cell quality, functional integrity, and yield—critical factors for the industrial production and clinical translation of cell therapy products.
2.6 Technical Optimization and Future Directions
Current T cell activation technologies offer significant room for advancement, with key future research directions including:
Novel Carrier Materials: Development of next-generation bead materials or alternative carriers to improve stimulation efficiency and reduce potential immunogenicity.
Cytokine Combination Optimization: Exploration of tailored cytokine cocktails to guide T cell differentiation toward desired phenotypes (e.g., memory T cells, effector T cells) and enhance persistence.
Precision Process Monitoring: Establishment of advanced monitoring systems (e.g., single-cell analysis, metabolite profiling) to enable real-time quality control during activation and expansion.
Mitigating Cell Exhaustion: Strategies to balance activation intensity with T cell exhaustion, aiming to generate long-lived, functionally robust T cells with improved therapeutic outcomes.
Personalized Protocols: Development of patient-specific activation regimens, tailored to individual immune profiles, to maximize treatment efficacy and minimize adverse events.
3. Research Significance
CD3 epsilon antibody-based magnetic bead activation technology addresses a critical need in both basic research and clinical translation by providing a reliable, scalable method for in vitro T cell activation and expansion. In basic immunology, it serves as a powerful tool to study T cell biology, signal transduction, and immune regulation. In clinical applications, it enables the standardized production of functional T cells for adoptive cell therapy, overcoming key technical barriers to the widespread availability of cell-based treatments for cancer and other diseases. By replicating physiological activation conditions, this technology ensures that in vitro-expanded T cells retain their natural functional properties, laying the groundwork for improved therapeutic outcomes and the advancement of precision medicine.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
T cell activation via CD3 epsilon antibodies follows a well-characterized signaling pathway: Binding of CD3 epsilon antibodies to the TCR complex induces conformational changes, triggering downstream signaling cascades (e.g., Zap70, Lck activation) that lead to transcription factor activation (e.g., NFAT, AP-1, NF-κB). Combined with CD28-mediated co-stimulation, this dual-signal activation promotes T cell proliferation, cytokine secretion, and differentiation into functional effector or memory cells. The magnetic bead platform enhances these mechanisms by mimicking the spatial organization of APC-T cell interactions, optimizing signal transduction efficiency.
4.2 Research Methods
Key research methods leveraging CD3 epsilon antibodies include:
Immunohistochemistry (IHC) and Immunofluorescence: For detecting CD3ε expression and T cell localization in tissue samples.
Flow Cytometry: For phenotypic analysis of T cell activation markers, subset distribution, and proliferation (e.g., CFSE labeling).
Magnetic Bead-Mediated Activation: Core method for in vitro T cell activation and expansion, as described in the SOP section.
Functional Assays: Cytotoxicity assays (e.g., ⁵¹Cr release assay, flow cytometry-based killing assays), cytokine secretion analysis (e.g., ELISA, intracellular staining), and proliferation assays (e.g., MTT, BrdU incorporation).
Gene Expression Analysis: RT-qPCR or RNA sequencing to study activation-related gene expression profiles.
4.3 Product Applications
ANT BIO PTE. LTD.’s CD3 epsilon antibodies, highlighted by the STARTER brand’s "S-RMab® CD3 epsilon Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2196), are indispensable tools for T cell research and clinical diagnostics:
T-Cell Lymphoma Diagnosis and Typing: Enables accurate diagnosis and differential diagnosis of peripheral T-cell lymphoma, angioimmunoblastic T-cell lymphoma, and other T-cell malignancies through specific CD3ε detection.
T-Cell Infiltration Assessment: Facilitates qualitative and quantitative analysis of T-cell infiltration in tumors, autoimmune diseases (e.g., rheumatoid arthritis), and transplant rejection tissues, supporting research on disease pathogenesis and treatment response.
Immune Microenvironment Research: Serves as a specific T cell marker to evaluate T cell density, distribution, and localization within tumor microenvironments, aiding in the development of immunotherapeutic strategies.
Thymic T-Cell Development Research: Used for identifying and studying T cells at different developmental stages in the thymus, advancing understanding of T cell maturation processes.
The S0B2196 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant rabbit monoclonal platform and validated for IHC, offers exceptional advantages: high specificity with clear membrane localization (ensuring reliable T cell identification in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community through the provision of high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in immunology, oncology, and cell therapy. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through cutting-edge life science research and clinical translation.
6. Related Product List
| Catalog No. | Product Name | Host |
| S0B2196 | S-RMab® CD3 epsilon Recombinant Rabbit mAb (SDT-R137) |
Rabbit |
| S0B2196 | S-RMab® CD3 epsilon Recombinant Rabbit mAb (SDT-R137) |
Rabbit |
| S0B1610 | CD3 epsilon Recombinant Rabbit mAb (Alexa Fluor® 488 Conjugate) (SDT-241-49) |
Rabbit |
| S0B2258 | CD3 epsilon Mouse mAb (SDT-570-36) | Mouse |
| S0B0212 | CD3 epsilon Recombinant Rabbit mAb (Alexa Fluor® 555 Conjugate) (S-241-49) |
Rabbit |
| S0B2132 | CD3 epsilon Recombinant Rabbit mAb (SDT-241-49) |
Rabbit |
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Optimizing In Vitro T Cell Activation and Expansion: The Pivotal Role of CD3 Eps 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026
- Eppendorf Collaborates with Dubai Police to Automate Forensics Laboratories 2/9/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/9/2026
- Eppendorf & Science Prize for Neurobiology 2026: Call for Entries! 2/4/2026
- Unraveling CD23’s Regulatory Role: Mechanisms in Immunoglobulin E-Mediated Aller 2/3/2026
- Deciphering the Diagnostic Value of CD21: Illuminating Follicular Dendritic Cell 2/2/2026


