Home > News > A Groundbreaking Study on Remodeling the Tumor Immune Microenvironment
A Groundbreaking Study on Remodeling the Tumor Immune Microenvironment
Dual-Defect Nitrogen-Rich Carbon Nitride-Based Heterostructural Nanocatalyst: A Groundbreaking Study on Remodeling the Tumor Immune Microenvironment
In the field of malignant bone tumor treatment, the efficacy of immunotherapy is often limited by issues such as low tumor immunogenicity, insufficient immune infiltration, and an immunosuppressive microenvironment. A recent study published in ADVANCED MATERIALS titled "Dual-Defect Nitrogen-Rich Carbon Nitride-Based Heterostructural Nanocatalyst for Improving the Therapeutic Efficacy of αPD-1 via Tumor Immune Microenvironment Remodelling" has successfully developed a novel dual-defect nitrogen-rich carbon nitride-based heterostructural nanocatalyst (CNO@CuMS), providing a brand-new approach to address this challenge.

In this study, Red Blood Cell (RBC) Lysis Buffer (abs9101) from Absin played a crucial role in facilitating the smooth progress of the experiment. The following is a detailed analysis combined with the experimental result figures.
Design and Performance Advantages of the Nanocatalyst
CNO@CuMS is composed of dual-defect nitrogen-rich carbon nitride (CNO) and copper-coordinated molybdenum disulfide nanosheets (CuMS). Through precise material design, it exhibits outstanding performance:
(1)Material Structure and Catalytic Activity
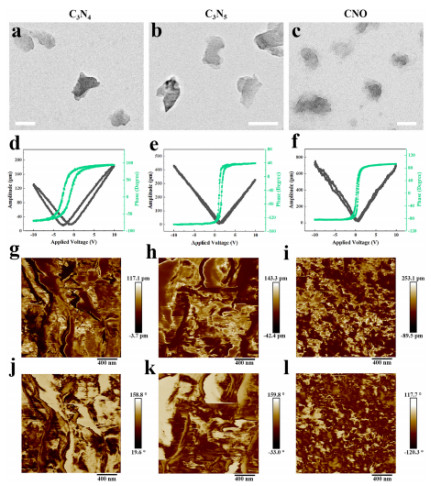
Figure 1
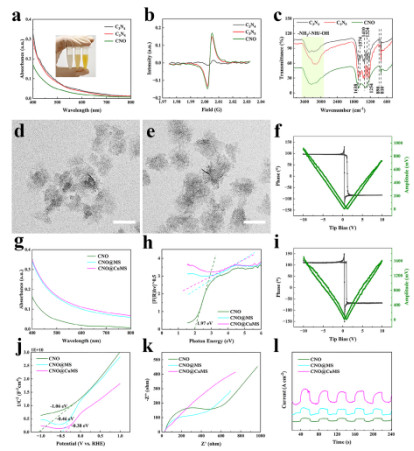
Figure 2
Transmission Electron Microscopy (TEM) images show that CNO nanosheets present an amorphous state due to nitrogen vacancies and oxygen doping (Figure 1c). Piezoresponse Force Microscopy (PFM) tests indicate that their piezoelectric amplitude (733.67 pm at -10 V) is significantly higher than that of C₃N₄ and C₃N₅ (Figures 1d-f), confirming that the introduction of defects can enhance piezoelectric performance. After forming a heterostructure with CuMS, the piezoelectric amplitude of CNO@CuMS is further increased to 1711.91 mV (Figure 2i). Additionally, Electrochemical Impedance Spectroscopy (EIS) shows that it has the smallest charge transfer resistance (Figure 2k), demonstrating its excellent charge separation and catalytic activity.
(2)Multimodal Reactive Oxygen Species (ROS) Generation
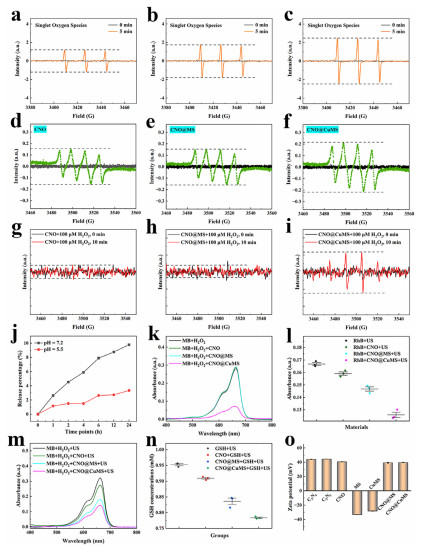
Figure 3
Electron Paramagnetic Resonance (EPR) tests confirm that CNO@CuMS can efficiently generate singlet oxygen (¹O₂), superoxide anions (・O₂⁻), and hydroxyl radicals (・OH) under ultrasonic treatment (Figures 3a-i). Moreover, it releases copper ions in an acidic environment (Figure 3j), which enhances ・OH generation through the Fenton-like reaction. Dye degradation experiments show that it can degrade 55% of methylene blue via a triple catalytic mechanism (Figure 3m), verifying its multimodal catalytic capability.
Tumor Killing and Immune Activation Mechanisms
CNO@CuMS triggers immunogenic cell death (ICD) through a dual mechanism of oxidative stress and cuproptosis, and remodels the tumor immune microenvironment:
(1)In Vitro Killing and ICD Triggering
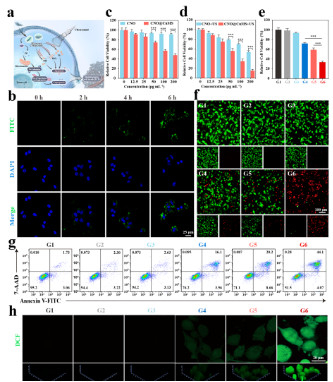
Figure 4
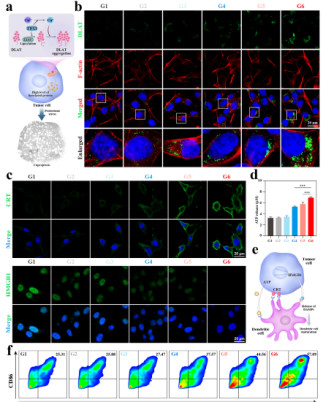
Figure 5
Cell Counting Kit-8 (CCK-8) experiments show that the survival rate of K7M2 cells in the CNO@CuMS + ultrasound group is only 35% (Figure 4d), and flow cytometry confirms that this group has the highest apoptosis rate (Figure 4g). Immunofluorescence results reveal that in this group, the exposure of calreticulin (CRT) on the cell surface and the release of high-mobility group box 1 (HMGB1) are the most significant (Figure 5c), and the ATP level is increased (Figure 5d), indicating that ICD is effectively activated.
(2)Immune Cell Regulation
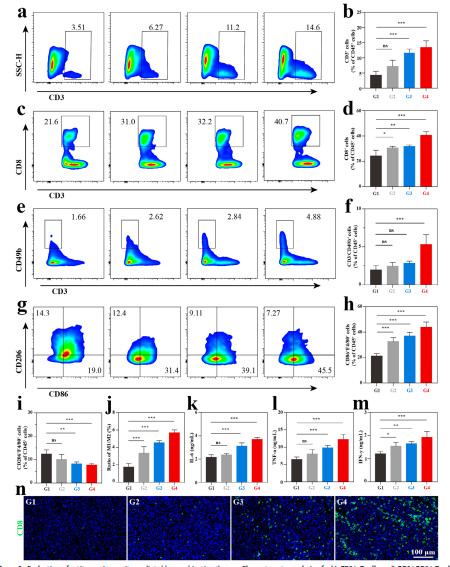
Figure 6
After treatment with CNO@CuMS + ultrasound, the expression of CD80 and CD86 in dendritic cells (DCs) is significantly upregulated (Figure 5f), promoting T cell activation. In animal experiments, the infiltration rate of CD8⁺T cells in the CNO@CuMS + ultrasound + αPD-1 group reaches 40.7%, and the ratio of M1/M2 macrophages increases to 6.3 (Figures 6c-j). Furthermore, the levels of pro-inflammatory cytokines (IL-6, TNF-α) are significantly elevated (Figures 6k-l), confirming the transformation of the immune microenvironment from an immunosuppressive to a pro-inflammatory state.
In Vivo Therapeutic Efficacy Verification
Animal experiments verify the efficient therapeutic effect of CNO@CuMS combined with ultrasound on orthotopic osteosarcoma:
Tumor Growth Inhibition
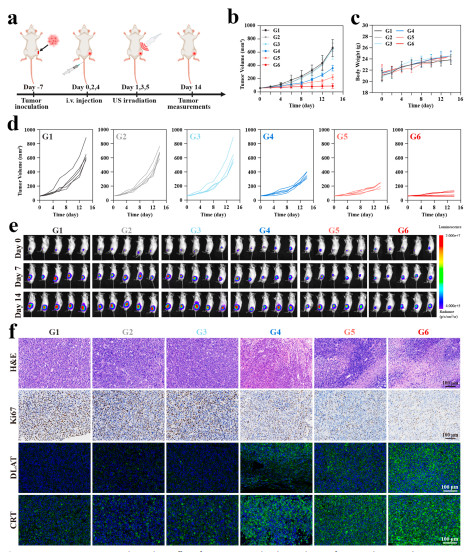
Figure 7
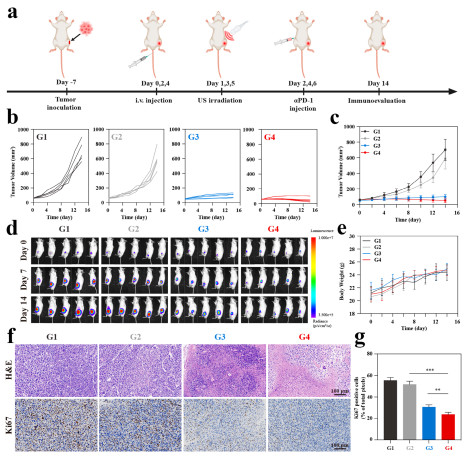
Figure 8
Bioluminescence imaging shows that the tumor fluorescence intensity in the CNO@CuMS + ultrasound group (G6) is the lowest (Figure 7e), and the tumor is almost completely inhibited after combining with αPD-1 (G4) (Figure 8d). Hematoxylin and Eosin (H&E) staining reveals that the G4 group has the largest tumor necrosis area and the fewest Ki67-positive cells (Figures 8f-g), with no obvious organ toxicity (Figures S51-S52).
Application of ABSIN Products
In the tumor immune cell analysis section, the study used RBC Lysis Buffer (abs9101) from Absin:
a. Application Scenario: Treating single-cell suspensions of tumor tissues to eliminate interference from red blood cells.
b. Functional Value: Tumor tissues, after digestion, are mixed with red blood cells, which affects the accuracy of flow cytometric analysis of immune cells. This buffer can specifically lyse red blood cells while preserving the activity of immune cells, ensuring the reliability of subsequent subset ratio analysis of CD3⁺T cells, CD8⁺T cells, natural killer (NK) cells, etc. (Figures 9a-f), and providing key support for the evaluation of the immune microenvironment.
Conclusion
This study achieves efficient remodeling of the tumor immune microenvironment by designing the CNO@CuMS nanocatalyst combined with ultrasound, significantly improving the therapeutic efficacy of αPD-1. In the experiment, the RBC Lysis Buffer (abs9101) from Absin provides important guarantee for the accuracy of immune cell analysis, demonstrating the key role of high-quality experimental reagents in ensuring the reliability of scientific research results. This study not only provides a novel nanocatalytic strategy for the clinical treatment of malignant bone tumors but also lays a foundation for the combined application of nanomaterials and immunotherapy.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


