Home > News > How Does the IFN-γ Detection Kit Ensure Quality Control of Cell Preparations?
How Does the IFN-γ Detection Kit Ensure Quality Control of Cell Preparations?
- Recent Advances
I. Why is IFN-γ Detection Crucial in Quality Control of Cell Preparations?
Interferon-gamma (IFN-γ), as a key Th1-type cytokine, plays a central role in immune regulation. In cell therapy products, the secretion level of IFN-γ is regarded as an important indicator for assessing the functional status of cells. Abnormal fluctuations in its expression often signal potential quality issues during the production or storage of cell products, including decreased cell viability, functional abnormalities, or contamination. Therefore, establishing an accurate and reliable IFN-γ detection method is of great significance for ensuring the quality stability and clinical safety of cell preparations.
Currently, Enzyme-Linked Immunosorbent Assay (ELISA) is the preferred method for quantitative detection of IFN-γ due to its high sensitivity and standardized operation. However, existing detection solutions still face many challenges: imported kits are expensive and have long delivery lead times, while some domestic kits lack sufficient accuracy and batch-to-batch consistency. These factors severely constrain the quality control and industrialization process of cell therapy products.
II. What Key Technical Specifications Must an IFN-γ Detection Kit Meet?
An ideal IFN-γ detection kit should possess excellent analytical performance characteristics. In terms of specificity, the kit needs to accurately distinguish IFN-γ from other common cytokines, such as Interleukin-2 (IL-2), IL-6, and IL-10, to avoid false-positive results caused by cross-reactivity. Regarding sensitivity, the lower limit of detection should reach the pg/mL level to meet the testing needs of samples with low analyte concentrations. The linear range needs to cover a concentration interval of 0-2000 pg/mL, ensuring that most samples can be tested directly without multiple dilutions, thereby reducing operational errors.
The stability and reproducibility of the kit are also key consideration metrics. The coefficient of determination (R²) of the standard curve should be greater than 0.99 to ensure the accuracy of quantitative results. The inter-assay coefficient of variation (CV) needs to be controlled within 10% to ensure the comparability of results from different kit batches. Furthermore, the stability of the standard directly affects the reliability of the detection results; liquid-form standards offer better stability and ease of use compared to lyophilized powders.
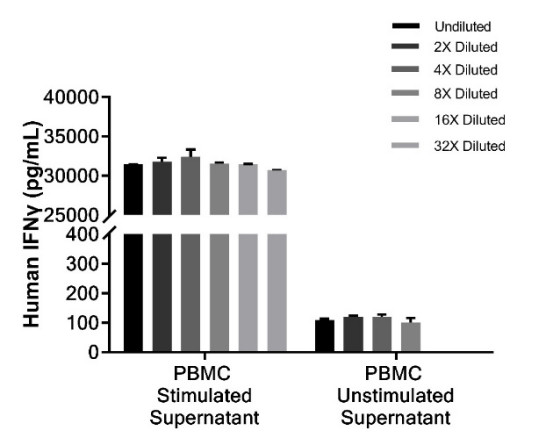
The performance verification of an IFN-γ detection kit should follow a systematic approach. In specificity verification, it is necessary to test the impact of high concentrations of interfering factors on the detection results to confirm the kit's specificity. Precision evaluation includes repeatability and intermediate precision experiments, quantifying the stability of the detection by calculating the coefficient of variation. Accuracy verification typically uses spike-and-recovery experiments, with recovery rates expected to fall within the acceptable range of 80%-120%.
Establishing the standard curve is a core part of performance verification. By testing a series of standard concentrations, a dose-response relationship model is constructed to evaluate the linear range and sensitivity. Stability studies for the kit also need to investigate the shelf life under different storage conditions, ensuring performance stability during transportation and use. Comparative studies with reference kits are also an important verification method, assessing the consistency of detection results through parallel testing of identical samples.
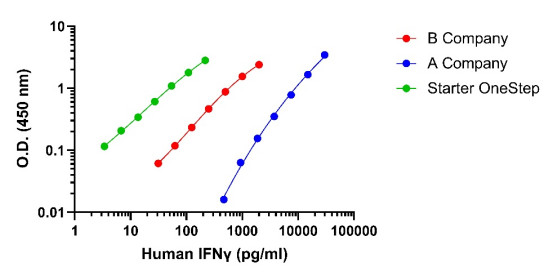
IV. What is the Application Value of IFN-γ Detection Kits in Cell Therapy?
In the field of cell therapy, IFN-γ detection kits have a wide range of application scenarios. During process development, monitoring IFN-γ secretion levels can help optimize culture conditions and stimulation protocols. In quality release testing, IFN-γ content is an important indicator for assessing the activity and function of cell products. Furthermore, in stability studies, changes in IFN-γ levels can reflect the quality changes of cell products during storage.
It is noteworthy that the kit's standard is traceable to international reference materials, such as those from the National Institute for Biological Standards and Control (NIBSC) in the UK, which guarantees the accuracy and comparability of the detection results. Regarding regulatory submissions, a fully validated IFN-γ detection method can meet the requirements of drug regulatory authorities for analytical methods, supporting the clinical research and marketing authorization applications of cell therapy products.
V. What is the Future Development Direction for IFN-γ Detection Kits?
With the rapid development of the cell therapy industry, higher demands are placed on IFN-γ detection kits. Future development trends include further improving detection sensitivity and specificity, developing multiplex detection solutions, and automating and standardizing the detection process. Additionally, the stability and user-friendliness of kits will continue to be optimized to meet the needs of different application scenarios.
Improving the quality control system requires joint efforts from industry, academia, and research. By establishing industry standards and methodological guidelines, the standardization process of IFN-γ detection technology can be promoted, ultimately providing reliable assurance for the safety and efficacy of cell therapy products. With continuous technological advancements, IFN-γ detection kits will play an increasingly important role in the quality control and industrial development of cell therapy.
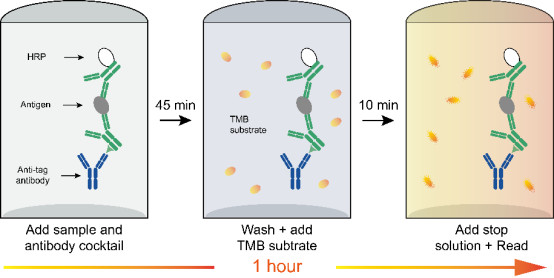
Hangzhou Start Bio-tech Co., Ltd.'s self-developed "Human IFN-γ OneStep ELISA Kit" is a detection kit characterized by high sensitivity, excellent reproducibility, and a convenient workflow. This product is ideal for applications in immune response research, infectious disease monitoring, cancer immunology, and drug efficacy evaluation.
Product Core Advantages:
* High Sensitivity & Convenient Operation: This product features an innovative one-step incubation design, reducing the total experimental procedure time from several hours (traditional methods) to just 90 minutes, while maintaining high sensitivity (detection limit can reach 0.5 pg/mL) and a broad detection range (1.56-100 pg/mL), significantly improving detection efficiency.
* Excellent Stability & Consistency: The core components of the kit undergo strict quality control verification, demonstrating excellent inter-batch consistency and stability. Both intra-plate and inter-plate coefficients of variation are less than 10%, ensuring the accuracy and reproducibility of experimental results.
* Suitable Key Application Scenarios:
This product is an ideal tool for conducting the following research:
Immune Response & Th1 Cell Function Research: For detecting IFN-γ levels in samples like serum, plasma, and cell culture supernatant to assess the level of cellular immune response.
Infectious Diseases & Vaccine Evaluation: For monitoring immune status during viral, bacterial, and parasitic infections, and for evaluating vaccine immunogenicity.
Tumor Immune Microenvironment Analysis: For assessing the functional status of immune effector cells like T cells and NK cells within the tumor microenvironment.
Autoimmune Diseases & Immunomodulation Research: For monitoring IFN-γ levels in autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease, and for evaluating the efficacy of immunomodulatory drugs.
* Professional Technical Support: We provide detailed product technical documentation, including standard operating procedures, sample pre-treatment recommendations, data analysis methods, and professional technical consultation, fully committed to assisting customers in efficiently completing accurate quantitative detection.
Hangzhou Starter Bio-tech Co., Ltd. (ANT BIO PTE. LTD. group member) is always dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "Human IFN-γ OneStep ELISA Kit" or to request a sample test, please feel free to contact us.
Product Information
| Catalog No. | Product Name | Size | Price |
| S0C3100 | Human IFN-γ OneStep OptiQuant Plus ELISA Kit | 96T | $500 |
| S0C3055 | Human IFN-γ OneStep HS ELISA Kit | 96T | $500 |
| S0C3040 | Human IFN-γ OneStep OptiQuant ELISA Kit | 96T | $500 |
| S0C3025 | Human IFN-γ Broad range ELISA Kit | 96T | $500 |
| S0C3005 | Human IFN-γ OneStep ELISA Kit | 96T | $500 |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


