Home > News > Can CD366 Become the Next Key Target in Cancer Immunotherapy?
Can CD366 Become the Next Key Target in Cancer Immunotherapy?
- I. Introduction: Why Explore New Immune Checkpoints?
The application of immune checkpoint inhibitors has significantly improved the treatment outcomes for various malignancies. Antibody drugs targeting Programmed Death Receptor-1 (PD-1), its ligand (PD-L1), and Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) have become a cornerstone of cancer immunotherapy. However, despite remarkable efficacy in some patients, challenges remain, including limited response rates, frequent drug resistance, and poor efficacy against "cold tumors." Therefore, there is an urgent need to explore new immune checkpoint targets to expand the scope and improve the effectiveness of immunotherapy. Among these, CD366 (also known as TIM-3, T-cell immunoglobulin and mucin-domain containing-3), as an emerging negative immune checkpoint molecule, has garnered widespread attention due to its unique role in tumor immune suppression. This article will systematically elaborate on the research progress of CD366 as a potential therapeutic target, covering its structural characteristics, expression patterns, mechanisms of action, and inhibitor development.
II. What are the Structural and Distribution Characteristics of CD366?
The human CD366 gene is located on chromosome 5q33.2, a region closely associated with various immune-related diseases such as asthma, allergies, and autoimmune diseases. CD366 is a type I transmembrane glycoprotein whose structure includes a signal peptide region, an immunoglobulin-like domain, a mucin-like region, a transmembrane domain, and an intracellular domain with tyrosine phosphorylation sites. Compared to other TIM family members, CD366's intracellular domain contains conserved tyrosine residues that play a key role in signal transduction.
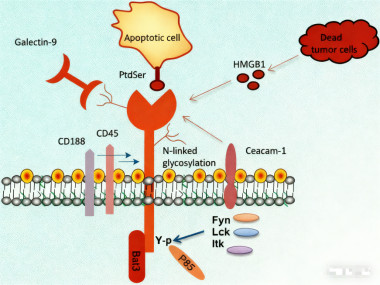
Initially identified as a surface marker on interferon-γ-secreting CD4+ Th1 cells and CD8+ cytotoxic T cells, subsequent research has shown that CD366 is also widely expressed on other immune cells, including regulatory T cells (Tregs), monocytes/macrophages, dendritic cells, and myeloid-derived suppressor cells (MDSCs). Ligands for CD366 include phosphatidylserine (PtdSer), galectin-9 (Gal-9), high mobility group protein B1 (HMGB1), and carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam-1). The binding of these ligands to CD366 participates in regulating various immune response processes, playing a particularly important role in the tumor microenvironment.
III. How Does CD366 Function in Tumor Immunity?
CD366 plays a critical role in tumor immune suppression. Its primary mechanisms include inhibiting T cell activation and proliferation, promoting immune tolerance, and mediating tumor immune escape. Specifically:
* Function in T Cells: In the tumor microenvironment, functionally exhausted CD8+ T cells highly express CD366 on their surface. The binding of Galectin-9 to CD366 induces phosphorylation of its intracellular tyrosine residues (e.g., Tyr256 and Tyr263), leading to the release of Human Leukocyte Antigen B-Associated Transcript 3 (Bat3) and inactive Lck, ultimately suppressing T cell effector functions. Furthermore, CD366 is also highly expressed on CD4+ FoxP3+ Treg cells and promotes the secretion of immunosuppressive factors such as interleukin-10, perforin, and granzymes, further enhancing the immunosuppressive microenvironment.
* Role in Myeloid Cells: CD366 is also expressed on CD11b+ Gr-1+ Myeloid-Derived Suppressor Cells (MDSCs). CD366+ T cells promote the proliferation of MDSCs in a Galectin-9-dependent manner, thereby suppressing local anti-tumor immune responses. Concurrently, CD366 is specifically upregulated on tumor-associated dendritic cells (TADCs), interfering with their recognition and presentation of DNA released by necrotic tumor cells, thus affecting the link between innate and adaptive immunity.
IV. What is the Development Progress of CD366 Inhibitors?
Currently, no CD366 inhibitor has been approved for marketing, but several candidate drugs globally have entered preclinical and clinical research stages. These drugs primarily include monoclonal antibodies, combination blockade strategies, and bispecific antibodies. The mechanism of action of CD366 inhibitors mainly involves blocking the interaction between CD366 and its ligands (such as Galectin-9), thereby reversing T cell exhaustion and restoring their anti-tumor function.
Notably, CD366 differs from classical immune checkpoints like PD-1 in its signaling pathway. CD366 does not rely on the Immunoreceptor Tyrosine-Based Inhibitory Motif (ITIM) for signal transduction; therefore, targeting CD366 may offer higher specificity and cause less interference with normal immune functions. Moreover, preclinical studies have shown that in PD-1 inhibitor-resistant models, CD366 expression is significantly upregulated on T cells. Combination therapy with anti-CD366 and anti-PD-1 antibodies can significantly enhance T cell proliferation and interferon-γ secretion, reverse the exhausted T cell state, and prolong survival.
Currently, several clinical trials are evaluating the safety and efficacy of CD366 inhibitors, both as monotherapies and in combination with PD-1/PD-L1 inhibitors. These combination strategies aim to overcome resistance to existing immunotherapies and improve tumor response rates and patient survival benefits through synergistic multi-target effects.
V. What is the Future Outlook for CD366-Targeted Therapy?
As an immune checkpoint molecule with high specificity, CD366 is selectively expressed in tumor tissues and plays a key role in regulating the immunosuppressive microenvironment and T cell exhaustion. With deepening understanding of its structure and function, CD366 has become an important research direction in the field of cancer immunotherapy. In the future, it is necessary to further explore the synergistic mechanisms between CD366 and other immune checkpoints (such as PD-1, CTLA-4) and to design more scientific, individualized treatment regimens. Furthermore, developing high-affinity, highly specific CD366 antibodies and optimizing combination therapy strategies hold promise for bringing new breakthroughs in cancer immunotherapy.
VI. Which Manufacturers Provide CD366 Antibodies?
Hangzhou Start Bio-tech Co., Ltd.'s self-developed "In Vivo Anti-Mouse CD366 (TIM-3) Recombinant Monoclonal Antibody" is an animal-grade immune checkpoint inhibitor characterized by high in vivo activity, extremely low endotoxin levels, and excellent stability. This product is ideal for preclinical studies in mouse tumor models, autoimmune diseases, and chronic infections.
Product Core Advantages:
* High In Vivo Activity & Specific Blockade: Validated by in vitro binding assays and in vivo tumor-bearing models, it efficiently blocks the interaction between TIM-3 and its ligands (e.g., Galectin-9, CEACAM-1), reverses T cell exhaustion, and restores anti-tumor immune function. It demonstrates significant tumor growth inhibition effects in various mouse tumor models.
* Extremely Low Endotoxin & Excellent In Vivo Compatibility: Endotoxin content is strictly controlled at <1.0 EU/mg, far below the requirement for in vivo experiments, minimizing non-specific immune activation caused by endotoxins and ensuring the accuracy and reliability of animal experiment results.
* Suitable Key Application Scenarios:
This product is an ideal tool for conducting the following in vivo studies:
Mechanistic Research in Tumor Immunotherapy: For evaluating the functional restoration of exhausted T cells and NK cells upon TIM-3 pathway blockade and its mechanism in reversing the tumor immunosuppressive microenvironment.
Combination Therapy Strategy Development: For exploring synergistic anti-tumor effects with other immune checkpoint inhibitors (e.g., PD-1/PD-L1) and developing effective combination treatment regimens.
* Chronic Infection & Viral Persistence Studies: For investigating the role of TIM-3 in T cell exhaustion and viral persistence during chronic viral infections (e.g., LCMV, HIV models).
* Validation in Autoimmune Disease Models: For assessing the protective role and therapeutic potential of the TIM-3 pathway in the pathogenesis and progression of autoimmune diseases (e.g., EAE, SLE models).
* Professional Technical Support: We provide detailed product technical documentation, including in vivo efficacy data, sterility and endotoxin test reports, recommended dosing regimens, and professional experimental design support, fully committed to assisting customers in achieving breakthroughs in immunology and translational research.
Hangzhou Start Bio-tech Co., Ltd. is always dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "In Vivo Anti-Mouse CD366 (TIM-3) Recombinant Monoclonal Antibody" or to request a sample test, please feel free to contact us.
Product Information
| Catalog Name | Product Name | Product Parameters |
| S0B1614 | Alexa Fluor® 488 Rat Anti-Mouse CD366 (TIM-3) Antibody (S-R478) | Host : Rat Conjugation : Alexa Fluor® 488 |
| S0B0953 | Rat Anti-Mouse CD366 (TIM-3) Antibody (S-R478) | Host : Rat Conjugation : Unconjugated |
| S0B1073 | Invivo Anti-Mouse CD366 (TIM-3) Recombinant mAb | Host : Rat Conjugation : Unconjugated |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


