Home > News > Bile Acid Receptor FXR Regulating Intestinal Epithelial Ferroptosis
Bile Acid Receptor FXR Regulating Intestinal Epithelial Ferroptosis
- Neonatal Necrotizing Enterocolitis (NEC) is a common life-threatening intestinal emergency in preterm infants. The incidence rate of NEC in very low birth weight infants worldwide is approximately 7%, and the mortality rate of cases requiring surgical intervention is as high as 80%. However, its pathogenesis has not been fully elucidated. Recently, the journal Immunity (IF=26.3) published the study "Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis", which reveals a novel mechanism by which the bile acid receptor FXR exacerbates NEC: it induces ferroptosis in intestinal epithelial cells (IECs) and inhibits the function of group 3 innate lymphoid cells (ILC3s). This finding provides a key target for clinical treatment. Absin multiplex Immunohistochemical Staining Kit (abs50013) played an important role in the analysis of tissue molecular localization in this study, helping the research team to clearly dissect the pathological regulatory network of the FXR-ferroptosis-ILC3 axis.

1. Research Background: Dilemmas in NEC Treatment and Scientific Questions
The core pathological features of NEC are intestinal barrier disruption and excessive inflammation caused by abnormal death of intestinal epithelial cells. Current treatments can only rely on symptomatic interventions such as fasting and antibiotics, and there is a lack of targeted therapeutic targets. Previous studies have suggested the following:
(1)Imbalance of intestinal flora in preterm infants (e.g., reduced number of short-chain fatty acid (SCFA)-producing bacteria) is closely associated with NEC, but how the flora regulates the function of intestinal immune cells remains unclear;
(2)Bile acid metabolism disorders may be involved in the pathogenesis of NEC. The bile acid receptor FXR is highly expressed in the intestinal epithelium of NEC patients, but its downstream mechanism of action is unknown;
(3)Ferroptosis (a type of iron-dependent, lipid peroxidation-driven cell death) is prone to occur in the intestines of preterm infants, but its association with NEC and the related regulatory pathways need to be verified.
Based on the above, the research team proposed the core scientific question: Does the intestinal flora induce NEC by regulating FXR, thereby mediating intestinal epithelial ferroptosis and abnormal ILC3 function?
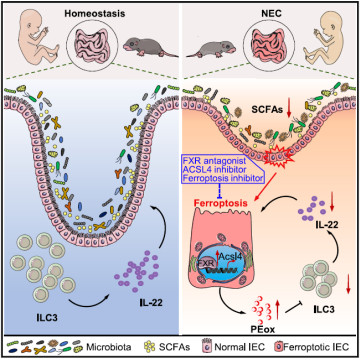
2. In-depth Analysis of the "Flora-FXR-Ferroptosis-ILC3" Axis
Following the classic scientific research logic of "clinical sample verification - animal model intervention - molecular mechanism exploration - therapeutic target verification", the research team revealed the new pathogenesis of NEC in four steps. Absin reagents provided technical support in key experimental links:
Step 1: Correlation Analysis of Clinical Samples - Identifying FXR as a Potential Biomarker for NEC
First, the study detected plasma samples from 46 neonates and intestinal tissue samples from 6 NEC patients, and the findings were as follows:
(1)The level of FGF19 (a product of FXR target gene) in plasma was negatively correlated with gestational age and birth weight (R=-0.6512/-0.6373), and positively correlated with the inflammatory marker CRP and the intestinal barrier injury marker I-FABP (R=0.5168/0.5904);
(2)The protein levels of FXR and FGF19 in intestinal epithelial cells (EpCAM+) of NEC patients were significantly increased (verified by immunofluorescence, Figure 1).
Key Reagent Support: The specific expression of FXR in the intestinal epithelium was confirmed by immunofluorescence staining, laying the foundation for focusing on "intestinal epithelial FXR" in subsequent mechanism studies.
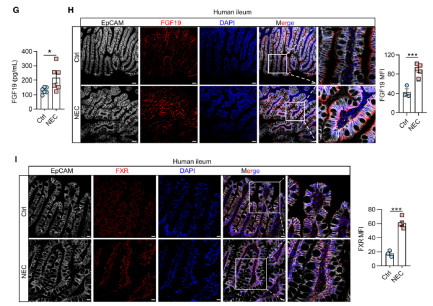
*Figure 2: Immunofluorescence staining of small intestinal tissues from NEC patients and control infants (EpCAM labels intestinal epithelium, FXR is red, DAPI stains nuclei). The left panel shows that the fluorescence intensity of FXR in the intestinal epithelium of NEC patients is significantly higher than that of the control group; the right panel verifies the difference through quantitative analysis (**p<0.001).
Step 2: Verification by Animal Models - Intestinal Epithelial FXR is a Key Pathogenic Factor in NEC
To clarify the functional role of FXR, the research team constructed the following models:
NEC mouse model: The clinical pathological environment of NEC was simulated through "flora colonization + hypoxia + low temperature + formula feeding";
Intestinal epithelium-specific FXR knockout mice (FxrΔIEC): Fxrfl/fl mice were crossed with Villin-Cre mice to achieve specific deletion of FXR in IECs.
The experimental findings were as follows:
(1)The expression of FXR in the intestinal epithelium of NEC model mice was 2.3 times higher than that of normal mice (verified by flow cytometry);
(2)The incidence of NEC in FxrΔIEC mice decreased by 58%, the intestinal inflammation score (villous necrosis, mucosal detachment) decreased significantly, and the survival rate increased to 75% (compared with 38% in wild-type mice);
(3)Supplementation of SCFAs (e.g., butyrate) could reverse the high expression of FXR in the intestinal epithelium of NEC model mice, suggesting a regulatory relationship of "flora-SCFAs-FXR".
Step 3: Exploration of Molecular Mechanisms - FXR Drives Intestinal Epithelial Ferroptosis via ACSL4
To analyze the downstream pathway of FXR regulating NEC, the study combined multi-omics and functional experiments:
(1)RNA sequencing (RNA-seq): By comparing IECs between FxrΔIEC mice and wild-type mice, it was found that differential genes were enriched in pathways such as "ferroptosis" and "lipid metabolism" (e.g., ACSL4, GPX4);
(2)Single-cell RNA sequencing (scRNA-seq): It was further identified that FXR mainly regulates ferroptosis-related genes in mature intestinal epithelial cells (C1/C2 clusters). The content of PE-PUFAs (lipid substrates for ferroptosis) in the intestinal epithelium of FxrΔIEC mice decreased by 40%;
(3)Verification of molecular mechanisms: ChIP experiments confirmed that FXR directly binds to the promoter region of ACSL4 (3 binding sites) and promotes lipid peroxidation through transcriptional activation of ACSL4. The use of the ACSL4 inhibitor PRGL493 can completely block FXR-induced ferroptosis in IECs.
Step 4: Extension of Pathological Effects - Ferroptotic Intestinal Epithelium Inhibits ILC3 Function via PEox
The study did not stop at the "FXR-ferroptosis" pathway, but further explored its impact on intestinal immunity:
(1)The number of ILC3s in the intestines of FxrΔIEC mice increased by 62%, and the secretion of IL-22 increased by 2.1 times (verified by ELISA). IL-22 can enhance intestinal barrier function by inducing Reg3b/Reg3g;
(2)Key finding: Lipid peroxides (e.g., PEox) released by ferroptotic IECs can inhibit the secretion of IL-22 by ILC3s in a dose-dependent manner (Figure 2). In vitro experiments showed that treatment with 10μM PEox can reduce the proportion of IL-22+ILC3s by 53%;
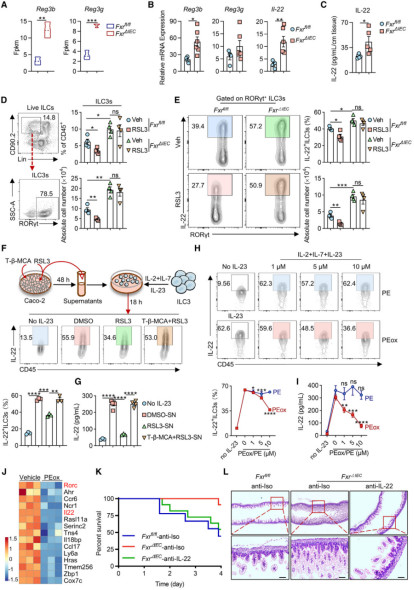
*Figure 2: Ferroptotic intestinal epithelial cells release oxidized phosphatidylethanolamine, thereby inhibiting IL-22 production by ILC3s
(3)Application of Absin Multiplex Fluorescence Immunohistochemistry Kit (abs50013): To clarify the co-localization relationship among FXR, ferroptosis marker (4-HNE) and intestinal epithelium, the study used Absin Multiplex Fluorescence Immunohistochemistry Kit (abs50013) to simultaneously detect EpCAM (intestinal epithelium), FXR (red) and 4-HNE (green) on the same tissue section. It was clearly observed that the co-expression level of FXR and 4-HNE in the intestinal epithelium of NEC patients was significantly increased (Figure 3), directly proving that FXR-positive intestinal epithelial cells are more prone to ferroptosis.
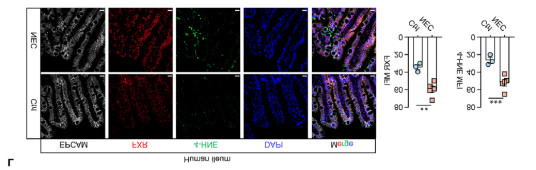
*Figure 3: Multicolor immunohistochemical staining results of abs50013 (small intestinal tissue from NEC patients). EpCAM (white, intestinal epithelium), FXR (red), 4-HNE (green, lipid peroxidation marker), DAPI (blue, cell nucleus). Yellow arrows indicate intestinal epithelial cells with co-expression of FXR and 4-HNE. The proportion of such cells in NEC patients is significantly higher than that in the control group (quantitative analysis in the right panel, **p<0.001).
Core Advantages of Absin Multiplex Fluorescence Immunohistochemistry Kit (abs50013)
| Product Advantages | Specific Roles (Combined with This Study) |
| Simultaneous detection of multiple targets | Four-color labeling of EpCAM (white), FXR (red), 4-HNE (green) and DAPI (blue) can be achieved with one staining, which clearly distinguishes intestinal epithelial cells from immune cells and eliminates non-specific signal interference |
| Low-background staining system | The kit contains specific blocking solution and signal amplification module, which can accurately capture the low-expression FXR signal in the complex microenvironment of intestinal tissue (containing mucus and flora) and avoid false negatives |
| Compatibility with multiple tissue types | It supports both frozen sections (for rapid detection of fresh intestinal tissue) and paraffin sections (for retrospective analysis of clinically archived samples). Both section types were used in the study, and the results showed high consistency |
| Friendly for quantitative analysis | The staining signal is stable, and the fluorescence intensity (e.g., MFI value) can be quantified by ImageJ. It was used in the study to statistically analyze the difference in the co-expression rate of FXR/4-HNE between NEC patients and the control group |
This study provides a new perspective on the pathogenesis of NEC and lays a foundation for clinical translation. In this breakthrough achievement, Absin Multiplex Fluorescence Immunohistochemistry Kit (abs50013), with its high specificity and ability of simultaneous detection of multiple targets, has become a core tool for dissecting the spatial correlation of "FXR-ferroptosis", helping the research team to clearly present the cellular localization and functional correlation of key molecules. In the future, Absin will continue to empower more scientific research in the fields of intestinal diseases and neonatal medicine with high-quality reagents and professional technical support.
Reference:
Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis. Immunity. 2025 Mar 1.
Product Used in the Article:
| Catalog | Product Name | Specification |
| abs50013 | Absin 5-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
Related products:
| Catalog | Product Name | Specification |
| abs50086 | Absin 2-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 100T |
| abs50087 | Absin 2-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 100T |
| abs50088 | Absin 3-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 100T |
| abs50089 | Absin 3-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 100T |
| abs50103 | Absin 3-Color IHC Kit B (Anti-Rabbit Secondary Antibody) | 100T |
| abs50104 | Absin 3-Color IHC Kit B (Anti-Rabbit and Mouse Secondary Antibody) | 100T |
| abs50012 | Absin 4-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50028 | Absin 4-Color IHC Kit(Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50167 | Absin 4-Color IHC Kit B (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50168 | Absin 4-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50013 | Absin 5-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50029 | Absin 5-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50014 | Absin 6-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50030 | Absin 6-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50048 | Absin 6-Color mlHC Kit(plus) (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50049 | Absin 6-Color IHC Kit (plus) (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50015 | Absin 7-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50031 | Absin 7-Color IHC Kit(Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50037 | Absin 7-Color IHC Kit (Anti-Rabbit and Mouse Secondary Antibody) | 20T/50T/100T |
| abs50038 | Absin 7-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50165 | Absin 7-Color IHC Kit (Anti-Rabbit Secondary Antibody) | 20T/50T/100T |
| abs50166 | Absin 7-Color IHC Kit (Anti-Rabbit&mouse Secondary Antibody) | 20T/50T/100T |
| abs50018 | Absin 10-Color IHC Kit | 100T |
| abs50083 | Lung Cancer Tumor Microenvironment mIHC Detection Kit (I) | 20T |
| abs50084 | Lung Cancer Tumor Microenvironment mIHC Detection Kit (II) | 20T |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


