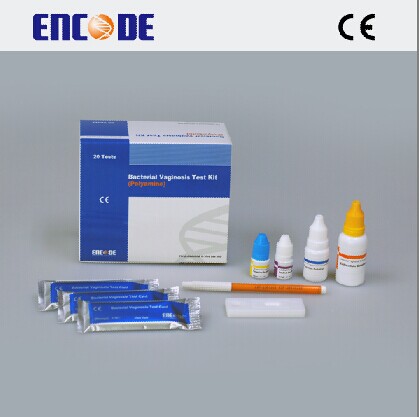
PACKING SPECIFICATION
20 tests per box
PURPOSE
For rapid diagnosis and treatment evaluation of Bacterial vaginosis.
OUTLINE
Bacterial vaginosis is actually a symptom-complex caused by the decrease of common bacterial population and the increase of anaerobic bacteria, e.g., Bacteroides, Mobiluncus spp, Gardnerella vaginalis, Prevotella spp, Peptococeus, and Mycoplasma hominis, etc. With a high morbidity (10-15%), Bacterial vaginosis is often attributed to habitual abortion, premature labor, premature breaking of fetal membrane, intrauteral infection, pelvic inflammation, cervical ulcer, and some other complicated diseases. The poly bacterial infection yields great difficulty to bacteriological diagnosis.
The standard BV laboratory diagnostic method "Amsel", so called gold standard, which is well recognized, covers the following items:
(1).PH>4.5 invagina
(2).Increased diluted vaginal secretion with peculiar smell
(3).Positive amine test
(4).The clue cell can be found in vaginal secretion smear.
The card is characterized by accuracy, simplicity, rapidity and sensitivity.
The correlation rate with Amsel reaches 92%, providing a satisfactory diagnostic result.
TEST PRINCIPLE
By use of double-membrane technique, the card is scientifically prepared on the basis of the amine test which specifically respondes in Bacterial vaginosis. Amine the pathogenic factor which exists in secretion of bacterial vaginitis is extracted with extracting fluid. After the extracted mixture added to the card sampling window, the amine signal is progressively amplified and finally appears on the card result window. The drawn yellow line turns blue purple. The operation of the assaying card is very simple. The whole test can be done with only one step and needs no special apparatus.
What the card assays is the final pathogenic substance directly leading to Bacterial vaginosis. The assaying pathogenic substance is also one of the diagnostic items rather than any other indirectly related enzymes or any other non-specific biochemical marker.
CONTENTS OF THE KIT
(1) BV assaying card 20/40pcs (2) BV extracting liquid 10ml×1 bottle
(3) BV reaction liquid 1 bottle (4) BV detecting pen l pcs
(5) Sampling pipette tips 20/40pcs (6) Instruction l pcs
(7) Negative quality control 1 bottle (8) Positive quality control l bottle
CAUTIONS
(1) For in vitro use only.
(2) Fresh sample is advisable, sealed sample can be kept for 48 hours at 4°C
(3) A bit more extracting liquid is needed while sample is too sticky.
(4) Owing to the double-membrane technique, that sample enters the assaying card from the sampling window cannot be seen from the result window.
(5) Carefully handle either sample or reagents because of some potential infectivity.
SAMPLING AND PROCESSING
Routinely take the vaginal secretion and quickly send it to the laboratory.
MATERIALS REQUIRED BUT NOT PROVIDED
Sampling swabs and test tubes
Pipette
Discarding materials barrel
SUCCESSIVE OPERATING PROCEDURES
(1). Add 3-5 drops of BV extracting liquid to the vaginal secretion-contained test tube, and then shake vigorously to mix thoroughly.
(2). Take out the assaying card. Add 3-5 drops secretion-extracting liquid mixture to the sampling window. Raise the end of sampling window end properly so as to make sample suck completely. Keep reacting completely.
(3). Add 2-3 drops of BV reaction liquid to the sampling window.
(4). Draw a line with BV detecting pen and observe the color change of the line within 2~3 minutes;
OBSERVE RESULTS
The test is positive while the yellow line in the result window turns blue purple and negative on little color alteration. Postponed observations are not advisable.
RESULT EXPLANATION
The shades of blue purple color in the result window represent the positive degrees and also suggest severity of the disease. The deep color means strong positive result and indicates severe disease while weak color means weak positive result and indicates slight disease. Sometimes the weak color is also due to over-diluting the vaginal secretion, medication and that the patient is under a subclinical status, etc.
PROBABLE INTERFERING FACTORS
(1). Using sample already diluted by physiological saline or some other solutions.
(2).Lower temperature especially in winter season possibly affects reading result.
(Suggestion: Raise the room temperature and lengthen the result reading time)
STORAGE AND PERIOD OF VALIDITY
Sealed and kept at room temperature (2-30°C), the kit is valid for one year.
bio-equip.cn