- Sepax Nanofilm SEC Columns

- Product Detail
- Company Profile
- High efficiency and resolution
- Extremely stable in high salt solution
- Great reproducibility
- Protein separation over very low salt concentration
- High protein recovery and maintaining biological activity
- Wide pH application range 2 - 9
Description
Utilizing proprietary surface technologies, Nanofilm® SEC phases are made of the uniform, hydrophilic, neutral, and polymeric thin films chemically bonded on the high purity and enhanced mechanical stability silica. Nanofilm® SEC phases have been innovatively and specially designed to ensure high resolution and maximum recovery for the separation of biological molecules at low salt concentration or organic buffers. The narrow dispersed, spherical silica particles of the Nanofilm® packings for SEC-150, SEC-250, and SEC-500 have nominal pore sizes at 150, 250, and 450 Å, respectively.
Packing
Nanofilm Technical Specifications
Phase
Nanofilm SEC-150
Nanofilm SEC-250
Nanofilm SEC-500
Material
Nanometer, hydrophilic film bonded silica
Particle size
5 µm
Pore size (Å)
~ 150
~ 250
~ 500
Protein MW range (native)
200 - 750,000
1,500 - 1,250,000
15,000 - 2,500,000
pH stability
2 - 8.5 (pH 9.0 can be tolerated temporarily)
Backpressure (psi for a 4.6×300mm)
~ 700
~ 700
~ 750
Maximum backpressure (psi)
~ 3,500
~3,500
~ 3,000
Salt concentration range
20 mM - 2.0 M
Maximum temperature (°C)
~ 80
Characteristic
Separation of 4 proteins and peptide by using a 4.6×250 mm SepaxNanofilm SEC-150 column.
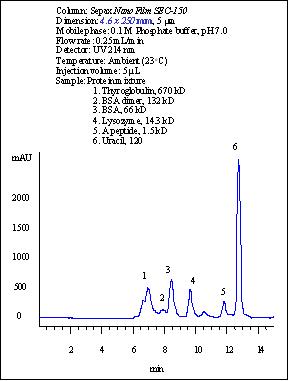 Four proteins (thyroglobulin, BSA dimer, BSA, lysozyme) and a peptide are well separated with 30,000 plates per meter for lysozyme. Even an impurity from lysozyme was very well separated. This was only seen separated with high efficiency capillary electrophoresis. (Reference: Sepax Capillary Electrophoresis Catalog). For standard test of size exclusion columns, manufacturers normally use Biorad standard proteins (thyroglobulin, IgG, ovalbumin, and myoglobin). The reason is that those proteins have pI less than 7.5, which are not positively charged at pH 7.0 separation condition. The highly positively charged proteins, such as lysozyme (pI=11.0), cytochrome C (pI= 10.6), aprotin (pI=11.0) are very difficult to elute due to the strong electrostatic interactions with the surface of the packing materials manufactured by other vendors. However, this is no longer a problem for Sepax Nanofilm SEC columns. Sepax sets its own standard of test proteins including lysozyme, a very sensitive protein for testing the quality of bioseparations.
Four proteins (thyroglobulin, BSA dimer, BSA, lysozyme) and a peptide are well separated with 30,000 plates per meter for lysozyme. Even an impurity from lysozyme was very well separated. This was only seen separated with high efficiency capillary electrophoresis. (Reference: Sepax Capillary Electrophoresis Catalog). For standard test of size exclusion columns, manufacturers normally use Biorad standard proteins (thyroglobulin, IgG, ovalbumin, and myoglobin). The reason is that those proteins have pI less than 7.5, which are not positively charged at pH 7.0 separation condition. The highly positively charged proteins, such as lysozyme (pI=11.0), cytochrome C (pI= 10.6), aprotin (pI=11.0) are very difficult to elute due to the strong electrostatic interactions with the surface of the packing materials manufactured by other vendors. However, this is no longer a problem for Sepax Nanofilm SEC columns. Sepax sets its own standard of test proteins including lysozyme, a very sensitive protein for testing the quality of bioseparations.
High Efficiency Separation
For biological separations, nonspecific interactions with the packing materials are the major contribution for low separation efficiency, such as peak tailing, as well as low recovery. The unique surface chemistry of Nanofilm SEC packings generates uniform polymer chains covalently bonded on the silica. Such a uniform stationary phase is neutral and hydrophilic, which has negligible nonspecific interactions with biological molecules, especially proteins. This special coating allows Nanofilm SEC columns to achieve high selectivity and high efficiency separations. For example, uracil, as a test compound could reach as high efficiency as 90,000 plates per meter, as shown in Table below.
Table 1. Column Efficiencies (Measured using uracil in 0.1 M phosphate buffer, pH 7.0)
| Nanofilm SEC-150 | >90,000 plates/meter |
| Nanofilm SEC-250 | >90,000 plates/meter |
| Nanofilm SEC-500 | >85,000 plates/meter |
Separation of BSA dimer from BSA by using a 4.6×300 mm Nanofilm SEC-250 column.
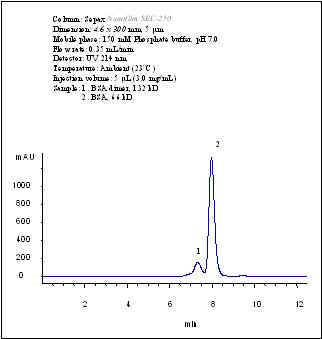 |
A Nanofilm SEC-250 column, 4.6×300 mm, achieved baseline separation of BSA dimer from BSA at 150 mM phosphate, pH 7.0. |
Separation of the aggregates from thyroglobulin
|
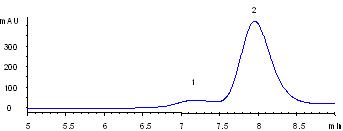 |
Thyroglobulin is a huge protein (MW 670,000). The commercial sample of thyroglobulin contains the aggregate impurity, possibly dimer or tetramer. Sepax Nanofilm SEC-500 provides a baseline separation of the aggregates from thyroglobulin.
The Impact of Pore Structure on Separation
The pore size of the SEC packings plays a critical role on protein separations. The selection of a Nanofilm SEC phase for a particular molecular weight range protein sample is based on the exclusion limit and linear molecular weight range of the Nanofilm packings that are shown below.
| Nanofilm Packings | Protein MW exclusion limit |
|---|---|
| Nanofilm SEC-150 | 750,000 |
| Nanofilm SEC-250 | 1,250,000 |
| Nanofilm SEC-500 | 2,500,000 |
Comparison of the separation patterns by Nanofilm SEC-150, Nanofilm SEC-250 and Nanofilm SEC-500.
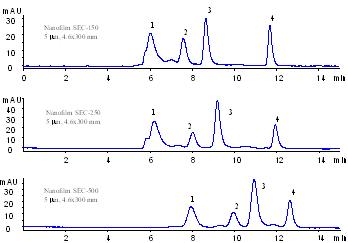 |
|
High Protein Recovery and Maintaining Activity
The Nanofilm SEC phases are composed of a hydrophilic and neutral polymer thin film with certain thickness that is chemically bonded on the silica surface. Proteins and other biological molecules have negligible nonspecific interactions with such stationary phases. The protein adsorption to the silica is suppressed, leading to high recovery and maintaining the activity after separations. Table below shows the recovery results of BSA and lysozyme, the representatives for acidic and basic proteins, separately.
|
Recovery of proteins from the Nanofilm SEC columns (measured with a 4.6×250 mm column in 0.15 M phosphate buffer, pH 7.0) | ||||||||||||||||||
High Stability of Sepax Nanofilm SEC Phases
The Nanofilm SEC columns uses full coverage bonded silica packing. The polymeric thin film is densely packed on the silica surface which greatly hinders the diffusion of the attacking molecules to the silica-polymer bonding, thus enabling exceptional high stability. The Nanofilm SEC phases are compatible with most aqueous buffers, such as ammonium acetate, phosphate, trizma and so on. When 150 mM phosphate buffer at pH 7.0 was used as the mobile phase to run the Nanofilm SEC columns, 1,000 injections or 3 months of usage made negligible deterioration for its performance. After 3 months of running in 150 mM phosphate buffer at pH 7.0, the average retention time change was within 5%.
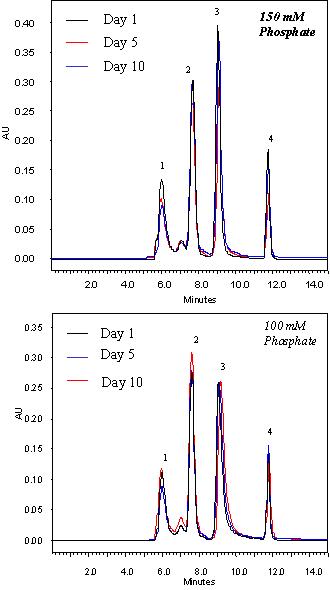 |
|
Figure above shows the separation profiles of a Nanofilm SEC-150 at the day 1, 5, and 10 with 10 hours/day running at the flow rate of 0.35 mL/min in 150 mM and 100 mM phosphate buffer, respectively. The separations are almost identical.
| Retention time variations of three proteins and uracil within 500 column volume injections. The test conditions are the same as those in Figure above. | 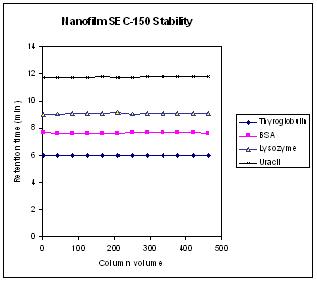 |
Figure above is the stability test for retention time of proteins and a small molecule over 500 column volume running in two weeks. It shows negligible retention time change for each of those three proteins as well as uracil within 500 column volume testing.
The Nanofilm SEC phases can tolerate very high concentration of salts, such as 1.0 M. The Nanofilm SEC columns are very stable in both organic solvents, such as methanol, ethanol, THF, DMF, DMSO, and so on, and the mixture of water and organic solvents.
The Nanofilm SEC columns have very good stability over a wide range of pH from 2 to 8.5. In addition, they can even tolerate very higher pH, such as pH 8.5-10 for temporary using.
The tolerance of temperature for the Nanofilm SEC phases is also excellent. The working temperature can go as high as 80 °C.
Reproducibility of the Nanofilm SEC columns
The controlled surface chemistry for synthesizing the Nanofilm SEC phases makes the surface coating very reproducible, leading to very consistent columns. The separation variation from batch to batch is within 5% for the retention time. Figure below is the separation of the Sepax standard protein mixture by the Nanofilm SEC-250 columns from two different lots. The largest variation is the retention time for lysozyme, which is ca. 2%.
Comparison of the separations by two different batches of Nanofilm SEC-250 (5 µm), 4.6×300 mm columns.
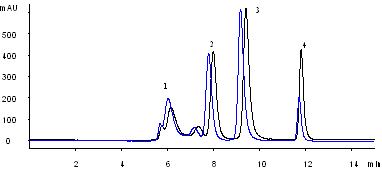
| Conditions | |
| Mobile phase: | 150 mM phosphate buffer, pH 7.0 |
| Flow rate: | 0.35 mL/min |
| Temperature: | Ambient |
| Injection volume: | 5 µL |
| Sample: | 1. Thyroglobulin (1.0 mg/mL) 2. BSA (1.0 mg/mL) 3. Lysozyme (1.0 mg/mL) 4. Uracil (0.1 mg/mL) |
MW Calibration for Protein Separation
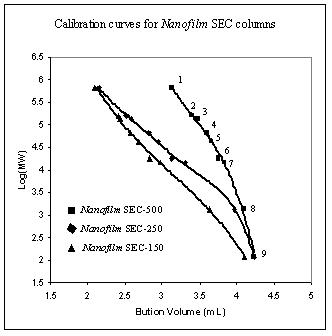
For size exclusion chromatography, individual pore size packings determine the range of molecular weight for separation, while the pore volume controls the separation capacity. Sepax's three pore size packings cover whole range of biological molecules. The protein calibration curves for Nanofilm SEC-150, Nanofilm SEC-250, and Nanofilm SEC-500 are shown below.
Protein MW calibration with Nanofilm SEC-150, Nanofilm SEC-250, and Nanofilm SEC-500 phases.
 |
|
Another widely used method to characterize the SEC phases is the calibration curve between protein MW and the distribution coefficient (Kd). Kd value is defined by the equation below:
Where Ve, VT, and Vo are the sample elution volume, the column total liquid volume, and the column void volume, respectively. The linear region between the protein MW and Kd is frequently used as the reference for the selection of the SEC phases. As plotted in Figure below, the Nanofilm SEC-150 phase has an excellent linear relationship between protein MW and Kd in a wide range of MW.
Protein MW calibration curve for Nanofilm SEC-150.
|
 |
Proteins Separations at very low salt concentration
In separation of positively charged proteins, a high concentration of salt is needed to shield the strong electrostatic interaction between the silica packing and the proteins. For example, cytochrome C, a highly positively charged protein (pI=9.6) is very difficult to be eluted at a low salt concentration, such as 50 mM phosphate buffer at pH 7.0. The need for high concentration salt to elute proteins greatly limits the applications. Sepax designed a special Nanofilm SEC phase that could well elute positively charged proteins. As shown in Figure below, a specially designed Nanofilm SEC-500 eluted cytochrome C at 50 mM, phosphate buffer at pH 7.0, which could not be done with any other silica based SEC phases. Such specially designed Nanofilm SEC phases can expand size exclusion chromatography to a variety of new applications, such as separation of salt sensitive biological molecules, protein binding studies, and LC/MS detections.
Cytochrome C elution profile with phosphate buffer at pH 7.0.
 |
|
-
Profile
Sepax Technologies, Inc., a privately held company, was founded in Delaware, USA in November 2002. It develops and manufactures HPLC consumables, bulk media, and equipment in liquid chromatography for chemical and biological separations. It is a fast growing technology company and owns patents, proprietary technologies and know-how. Sepax has emerged as a leader in the biological separation industry in the global market.
Business strategies
Sepax Technologies, Inc. develops innovative HPLC consumables, bulk media, and instruments to solve separation challenges in the global market. We provide solution-based products by closely working with our customer scientists in pharmaceuticals, biopharmaceuticals, research institutes and government labs. Our strong technical team develops various methods with our customers to analyze complex biological molecules. As a leader in the biological separation industry, we constantly develop the best bio-separation technologies and products for our global customers. We have reached a competitive position globally in fast growing regions.
Vision Statement
To become a technology leader in the biological separation industry in the global market and provide solution based products and services for our customers.
Operation
Sepax Technologies, Inc.
Our company headquarter in Delaware is located in Delaware Technology Park with facilities of 15,000 ft2 dedicated to the development of separation resin technologies and instrumentation, production of HPLC resins, columns and CE consumables. It is also the marketing and sales center for our US and global markets.
Sales
Domestic
Audrey Fisher
Field Sales Account Manager
(Northern CA areas, UT, CO, OR)
Direct Phone: 925-324-5223
Email: afisher@sepax-tech.com
Kathleen Falls
West Coast Regional Manager
(All other CA areas, WA, TX, AZ, NM, MI)
Direct Phone: 323-228-0004
Email: kfalls@sepax-tech.com
Michael Hunnewell
Field Sales Account Manager
(MA, RI, NH, CT, NY)
Direct Phone: 302-650-3955
Email: mhunnewell@sepax-tech.com
Colleen Callahan
Midwest Account Manager
(WI, IL, IN, OH, MO)
Direct Phone: 302-366-1101, ext 106
Cell Phone: 302-339-8747
Email: ccallahan@sepax-tech.com
All other areas, please contact
Helen Gu
Vice President, Sales
Direct phone: 302-650-3909
Email: hgu@sepax-tech.com
International
Tingting Wu
Executive Account Manager/Marketing
Phone: 302-366-1101, ext 115
Email: twu@sepax-tech.com
| Request Information |
| Other Products |
| Related Products |
| Recently viewed products |
- SiteMap