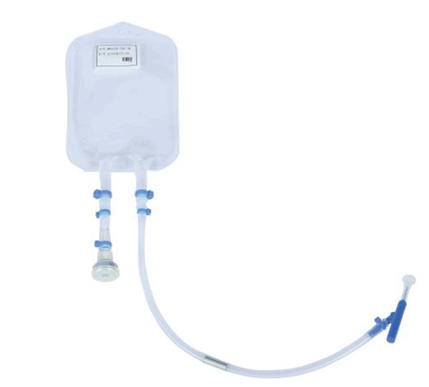
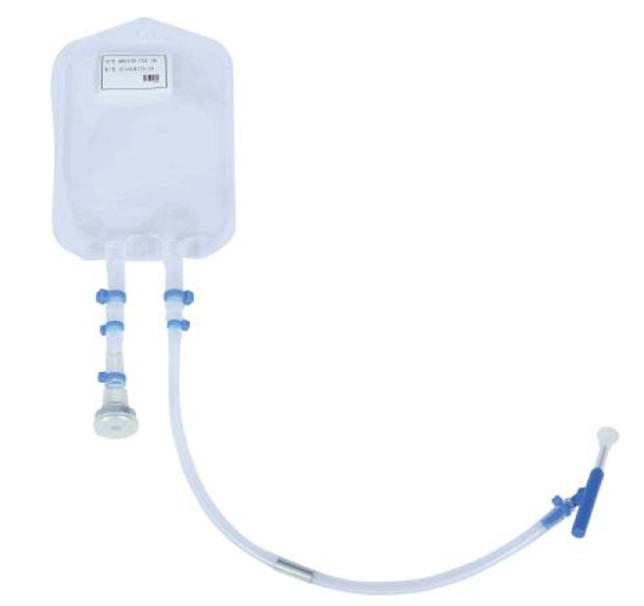
| specifications |
5 mL-1L,Other specifications can be customized |
| Usage temperature |
-80°C~60°C |
| Sterilization method |
Gamma ray irradiation sterilization (25-40 kGy) |
| Packaging form |
Double layer PE bag vacuum packaging |
| Membrane material information |
| structure |
LDPE/EVOH/ULDPE(Liquid contact layer) |
| thickness |
0.325 mm |
| compliance |
ISO 10993-4: Hemolysis hemolysis test
ISO 10993-5: Cytotoxicity of Cytotoxicity
IS0 10993-6: lmplanting test Implantation test
ISO 10993-10: Sensitization and sensitization tests
ISO 10993-11: Acute Systemic Toxicity Test
USP<85>: Bacterial endotoxins LAL test for bacterial endotoxins
USP<88>: Biological reactivity testing, in vivo, classV in vivo biological reactions
USP<661>:Plastic Containers
European Pharmacopoeia tests, Ch.3.1.5 Plastic containers European Pharmacopoeia tests Chapter 3.1.5
ADCF: No animal derived ingredients |