Overall Dimensions:
LFGI-R: 36″ wide ・ 34″ deep ・ 79.5″ high
Work Area Dimensions:
LFGI-R: 32″ wide ・ 24″ deep ・ 27″ high
Designed to fit through standard door openings and elevators.
Overall Dimensions:
LFGI-R: 36″ wide ・ 34″ deep ・ 79.5″ high
Work Area Dimensions:
LFGI-R: 32″ wide ・ 24″ deep ・ 27″ high
Designed to fit through standard door openings and elevators.
Compounding Aseptic Containment Isolators: USP 797 Regulations for Compounded Sterile Preparations
USP 797 provided the first official and enforceable requirements for preparing CSPs -Compound Sterile Preparations. USP 797 is the U.S. Pharmacopeia’s (USP) Revised General Chapter for Pharmaceutical Compounding Sterile Preparations. According to the organization these requirements set “practice standards to help ensure that compounded sterile preparations are of high quality”. Chapter fundamentally changed the way that facilities that prepare compounded sterile preparations approach their work.
USP 797 applies to the diverse range of facilities that prepare Compounded Sterile Preparations
Chapter applies to all facilities that prepare CSPs. This includes hospital and health-system pharmacies that prepare compounded sterile preparations including main hospital pharmacy operations and satellite pharmacy units. USP 797 also applies to clinics, hospital care units as well as other facilities that handle the compounding of sterile preparations. As facilities change their procedures to meet USP 797 requirements, they are finding that barrier isolators can provide an ideal alternative to a more costly cleanroom.
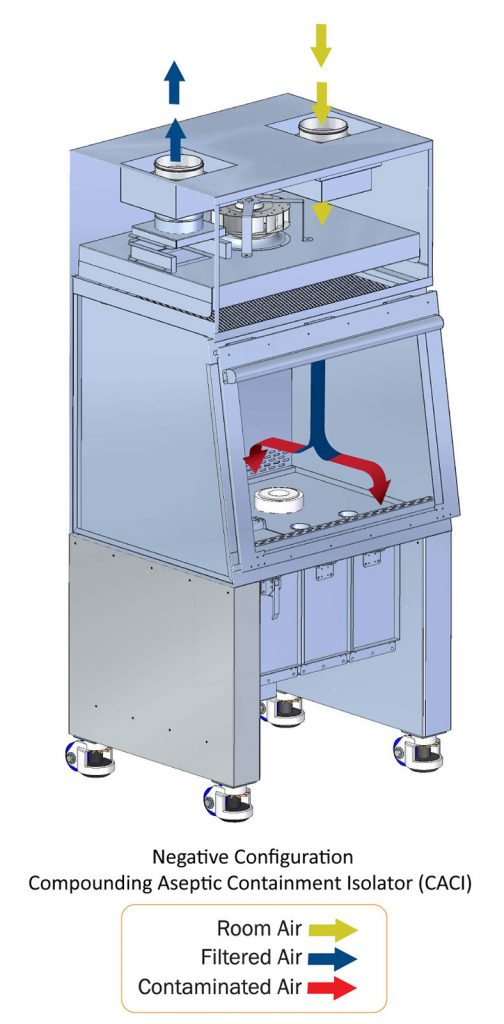
The Food and Drug Administration states that a barrier isolator is: “a decontaminated unit supplied with HEPA filtered air that provides uncompromised continuous isolation of its interior from the external environment, including surrounding cleanroom air and personnel.” Installing a Compounding Aseptic Containment Isolator provides cleanroom conditions within a contained workspace. Compounding Aseptic Containment Isolators provide an ISO Class 5 (Class 100) environment for product preparation, with work occurring inside a closed, pressurized work space, accessible only via a sealed gloves system.
GENERAL STANDARDS FOR USP 797 COMPOUNDING ASEPTIC CONTAINMENT ISOLATORS:
Germfree’s Compounding Aseptic Containment Isolators are an ideal solution for providing a clean work environment in the pharmacy industry when compounding sterile preparations.
As there no true uniform industry standards for manufacturing isolators, there are significant design differences among manufacturers. A well-designed isolator that will significantly reduce microbial contamination should meet a range of minimal standards. Germfree’s Radiopharmacy Shielded Isolators meet or exceed these standards:
- Unidirectional airflow showers the work zone with a continuous supply of filtered air that sweeps contaminants out through the air exhaust system.
- Negative air pressure maintains containment of hazardous substances within the isolator.
- Easy to clean and disinfect inside and out.
- Pass-through system that isolates the interior of the unit from the room when materials are transferred in and out.
- Made of durable materials (stainless steel, glass, and high-performance, scratch-resistant plastics)
- Includes height adjustors to make the work environment ergonomic.