Home > News > FDA Issued an EUA for NEST Disposable Sampler
FDA Issued an EUA for NEST Disposable Sampler
- On the evening of July 30, NEST Biotech was notified that the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the emergency use of its product — disposable sampler (VTM).
It makes NEST the only non-US company being authorized overseas.
After being granted emergency use authorization by the FDA, the product was requisitioned by the US Department of Health & Human Services and distributed to testing agencies in 50 states for use! It was also authorized to be sold in other countries that accept FDA’s emergency use authorization.
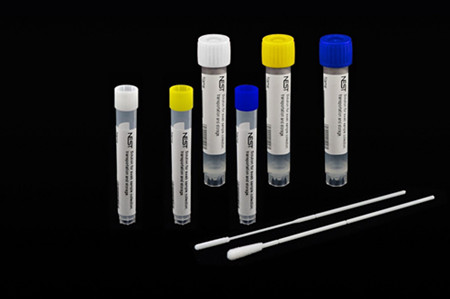
In view of the current Sino-US relations, FDA has done many investigations and tests and finally issued an EUA for NEST disposable sampler, for which we’ve also have made tremendous efforts.
High-standard work flow:
Class 10,000 clean rooms
Liquid filtration and sterilization system
Automatic filling system
Enhanced security with irradiation sterilization
Strict and improved inspection
……

Develop a high-standard work flow!
https://www.youtube.com/watch?v=L5fpzoko4jw
Details allow you to recognize the whole through observation of any part.
https://www.youtube.com/watch?v=M1SKMJCxh5U

Years of experience in injection molding: backed by Vanguard, we have natural advantages in injection molding. We offer more specification and dimension options to better meet your needs, while guaranteeing product quality.
Guaranteed sterility: Rhodotron TT20 electron accelerator from Belgium-based IBA, being safe and environment-friendly in operation and free of chemical residues, features less time consumption and good effect.
All products are in stock. Please contact our local agents or distributors as necessary.
https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/faqs-viral-transport-media-during-covid-19?from=singlemessage#VTM
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- CD182 Antibodies: Deciphering CXCR2’s Multifaceted Roles in the Tumor Microenvir 1/29/2026
- Lifeasible Launches Enhanced Bulk Segregant Analysis Service to Boost Plant Bree 1/29/2026
- Eyestem Receives CDSCO Approval to Initiate Phase 2 Randomized, Controlled Human 1/28/2026
- CD172a Antibodies: A Cornerstone Tool for Immune Regulation Research 1/27/2026
- CD170 Antibodies: Blocking Myeloid-Derived Suppressor Cell-Mediated Tumor Immune 1/26/2026
- CD163 Antibodies: Targeted Delivery Systems for Promoting Vascular Regeneration 1/25/2026
- CD14 Antibodies: Deciphering Dual Regulatory Mechanisms in Inflammasome Activati 1/24/2026
- CD146 Antibodies: Targeting Lipid Metabolism and Energy Homeostasis to Intervene 1/23/2026
- Be Prepared for 2026 Weather Extremes Says Cold Chain Technologies 1/23/2026


