Home > News > MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity
MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity
Preclinical data in rats demonstrate dose-dependent and rapid brain targeting of intranasal semaglutide with low systemic exposure
EMMETTEN, Switzerland, January 22, 2026 / Biotech Newswire / -- MetP Pharma AG, a pioneer in nose-to-brain drug delivery, today announced new preclinical data demonstrating that its proprietary nasal technology achieves rapid, dose-dependent brain targeting of GLP-1–based drugs such as semaglutide while maintaining low systemic exposure.In a 2025 preclinical study in rats, intranasal administration of semaglutide using MetP Pharma’s technology resulted in brain-to-plasma ratios consistently greater than 1, confirming preferential delivery to the central nervous system (CNS). Brain exposure increased proportionally with dose and was observed rapidly after administration, highlighting the robustness and controllability of the platform.
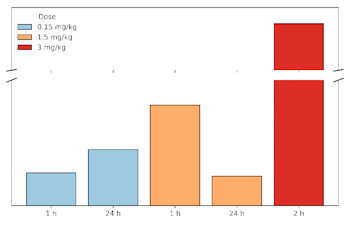
Figure: Brain-to-plasma ratios at different time points following nasal administration of three doses of intranasal semaglutide (brain values represent one hemisphere).
A brain-to-plasma ratio > 1 for semaglutide has never been described with subcutaneous/oral/nasal administration and is extremely unusual - according to current knowledge, it is almost impossible.
In contrast, preclinical studies in rats following subcutaneous or oral semaglutide administration demonstrate only a very limited brain penetration (Kp < 0.0005 to < 0.01) (1,2). A brain-to-plasma ratio > 1 with MetP Pharma’s nasal technology suggests that semaglutide is actively accumulated in the brain leading to strong central satiety and appetite suppression.
“These data reinforce our belief that effective CNS targeting is achievable without high systemic exposure,” said Dr. Claudia Mattern, Chief Scientific Officer of MetP Pharma. “By shifting GLP-1 exposure toward the brain and away from the periphery, our technology directly addresses key limitations of current injectable and oral GLP-1 therapies.”
A next-generation GLP-1 strategy
Current subcutaneous and oral GLP-1 receptor agonists generate high plasma levels but achieve only limited direct brain exposure, contributing to dose-limiting gastrointestinal side effects and restricting their broader use in CNS-driven indications. MetP Pharma’s nasal enabling technology is non-invasive, designed for easy self-administration, and leverages direct nose-to-brain transport pathways that bypass the blood–brain barrier to overcome these limitations.
The platform enables several strategic opportunities:
Lifecycle extension and differentiation
Brain-targeted reformulations of established GLP-1 assets offer a clear path to differentiation with regard to injectable and oral competitors and a practical strategy to extend product lifecycles.
Improved benefit–risk profile
Targeted CNS exposure combined with less dosing may enhance efficacy in obesity and addiction while mitigating gastrointestinal side effects that currently limit patient adherence and dose escalation.
Scalable platform across CNS indications
Beyond obesity, the technology is applicable to addiction disorders (including alcohol, substance, and food addiction) and other CNS-driven diseases, creating multiple partnering opportunities across metabolic and neuropsychiatric portfolios.
Building on a validated nose-to-brain platform
The new in vivo study results build on MetP Pharma’s extensive preclinical body of evidence demonstrating rapid and sustained CNS exposure of GLP-1–based therapeutics using its proprietary nasal technologies. Together, these results position MetP Pharma’s platform as a versatile enabling solution for the next generation of CNS-active metabolic drugs.
“GLP-1 biology is fundamentally linked to the brain,” Dr. Mattern added. “Our approach is designed to unlock that potential more directly, opening new therapeutic and commercial possibilities for well-validated molecules.”
MetP Pharma is actively exploring partnerships to advance brain-targeted GLP-1 programs into further preclinical and clinical development.
About MetP® Pharma AG
MetP® Pharma AG is an independent pharmaceutical R&D and product development company based in Switzerland, specializing in nose-to-brain drug delivery. The company’s proprietary MetP® Technology is a comprehensively patented platform that enables direct, efficient delivery of therapeutics to the brain, bypassing the blood-brain barrier.
The brain-targeting capabilities of MetP® Technology have been validated by several independent research institutions worldwide and are featured in 67 predominantly peer-reviewed publications. The platform has proven its clinical utility in several preclinical and clinical trials and has demonstrated to be fast, safe, effective, user-friendly, and cost-efficient.
MetP® is actively advancing its own pipeline of intranasal therapeutics targeting conditions such as concussion, insomnia, ADHD, multiple sclerosis (MS), and hypogonadism. A strong global intellectual property portfolio protects the platform until 2037/2039.
Beyond formulation, MetP® offers an integrated, end-to-end solution including its proprietary unit-dose applicator and industrial-scale filling technology - providing partners with a fully developed, patent-protected, and ready-to-use drug delivery system.
Contact
MetP Pharma AG
Dr. Claudia Mattern
CSO
+41-41-618 30 30
info@mattern-pharma.com
References:
Lee TS, Park EJ, Choi M, Oh HS, An Y, Kim T, Kim TH, Shin BS, Shin S. Novel LC-MS/MS analysis of the GLP-1 analog semaglutide with its application to pharmacokinetics and brain distribution studies in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Apr 15;1221:123688. doi: 10.1016/j.jchromb.2023.123688. Epub 2023 Mar 22. PMID: 36989942.
Abdulhameed N, Babin A, Hansen K, Weaver R, Banks WA, Talbot K, Rhea EM. Comparing regional brain uptake of incretin receptor agonists after intranasal delivery in CD-1 mice and the APP/PS1 mouse model of Alzheimer's disease. Alzheimers Res Ther. 2024 Aug 1;16(1):173. doi: 10.1186/s13195-024-01537-1. PMID: 39085976; PMCID: PMC11293113.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- CD146 Antibodies: Targeting Lipid Metabolism and Energy Homeostasis to Intervene 1/23/2026
- Be Prepared for 2026 Weather Extremes Says Cold Chain Technologies 1/23/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026


