Home > News > Takara Bio Europe Launched of New High Quality (HQ) Grade mRNA Production Enzymes "T7 RNA Polymerase
Takara Bio Europe Launched of New High Quality (HQ) Grade mRNA Production Enzymes "T7 RNA Polymerase
Launch of high-quality (pre-clinical) enzymes suitable for pre-clinical and process development in mRNA vaccine and therapeutics


(Left) Pyrophosphatase (inorganic), HQ; (Right) T7 RNA Polymerase, HQ.
Saint-Germain-en-Laye, France – September 12, 2023 – Life Science Newswire – Takara Bio Europe (TBE) announced today the launch of new High Quality (HQ) grade mRNA production enzymes "T7 RNA polymerase, HQ" and "Pyrophosphatase (inorganic), HQ" suitable for pre-clinical and process development in mRNA vaccine and therapeutic manufacturing.
HQ grade enzymes have features more similar to GMP grade quality (e.g. the same set of quality tests as GMP grade, see details below) than RUO grade enzymes, facilitating a smooth transition to GMP-grade manufacturing. Additionally, the high volume format (1 mL/tube) offers convenience for scaling up processes.
We are expanding our lineup of HQ enzymes to support pre-clinical and process development stages in mRNA production, including another HQ grade product "Recombinant RNase Inhibitor, HQ", set to be launched soon. We also plan to sequentially provide GMP grade enzymes suitable for active pharmaceutical ingredients for manufacturing mRNA vaccines and therapeutic drugs.
GMP grade reagents are manufactured and quality controlled in accordance with the GMP guidelines of PIC/S (Pharmaceutical Inspection Agreement and Pharmaceutical Inspection Joint Scheme). GMP grade reagents are suitable for manufacturing active pharmaceutical ingredients for clinical trials.
About Takara Bio
Takara Bio is a renowned supplier of high-quality life science products to support various areas of research, including genomics, molecular and cellular biology. Our portfolio includes products such as PCR reagents, cloning systems including the In-fusion seamless cloning system, vaccine development, pathogen detection, gene expression and viral vector tools and kits, and protein purification reagents. With an extensive range of research and GMP-grade products, Takara Bio caters to the diverse needs of researchers in academia, industry, and clinical settings with its. Takara Bio's products are designed to enable reliable, accurate, and efficient research across a range of applications, making them essential tools for cutting-edge research in the life sciences.
In addition, Takara Bio. serves as a one-stop CDMO providing a wide range of mRNA vaccine and therapeutic development, manufacturing, and quality testing services with the aim of enhancing the reach of mRNA as a novel modality. For more information, please click here.
Product Summary
*HQ grade
HQ grade reagents match GMP grade in quality standards, using the same raw materials and procedures. They are ideal for pre-clinical tests and process development of mRNA manufacturing due to identical quality tests to GMP grade including animal- and human-origin and β-lactam (antibiotics) free
** GMP grade
GMP grade reagents are manufactured and quality controlled in accordance with the GMP guidelines of PIC/S (Pharmaceutical Inspection Agreement and Pharmaceutical Inspection Joint Scheme). GMP grade reagents are suitable for manufacturing active pharmaceutical ingredients for clinical trials.
Product information
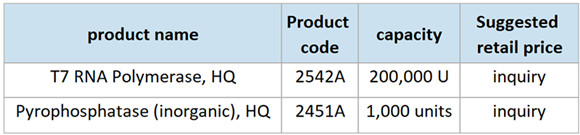
*** T7 RNA Polymerase, HQ (Product code: 2542A)
Capacity: 200,000 U
https://www.takarabio.com/products/mrna-and-cdna-synthesis/in-vitro-transcription/rna-polymerases-and-rntps/t7-rna-polymerase-hq
**** Pyrophosphatase (inorganic), HQ (Product code: 2451A)
Capacity: 1,000 U
The page goes live on 9/11/2023
Contact Information
Pierre Lacaze
Managing Director
34 Rue de la Croix de Fer CS 30158
78100 Saint-Germain-en-Laye
France
ivd_eu@takarabio.com
+33 1 39 04 68 80
Related News
- Ultra Smooth Hyperbolic Mirrors 2/9/2026
- Low Binding DNA Consumables for NGS and Molecular Biology 1/30/2026
- Creative BioMart Launches GenePowerTM Controlled Randomization to Support Precis 1/29/2026
- Biotech Fluidics: Instrument Optimized Vacuum Degassers. 1/28/2026
- High Speed Framing Cameras for Fusion Research 1/21/2026
- Tri-coded Storage Tube Optimized for Maximum Sample Recovery 1/16/2026
- Optimised UV Lenses for Forensic Investigation 1/16/2026
- Large Format Aerial Surveillance Lens 1/7/2026
- Breathing New Life into Older High-speed Cameras 1/7/2026
- New Handheld Decapper Boosts Speed and Consistency of 48-Format Tube Handling 1/6/2026


