Home > News > Reshaping Hematologic Malignancy Treatment: Multi-Mechanism Synergy of CD38 Antibodies
Reshaping Hematologic Malignancy Treatment: Multi-Mechanism Synergy of CD38 Antibodies
- 1. Concept
CD38 is a 46 kDa type II transmembrane glycoprotein, structured with an N-terminal short cytoplasmic tail, a single transmembrane domain, and an extended C-terminal extracellular region. As a bifunctional ectoenzyme, it possesses both cyclase and hydrolase activities, serving as a central regulator in nucleotide metabolism. Using NAD⁺ as a substrate, CD38 catalyzes the production of cyclic ADP-ribose (cADPR) and other metabolites—key second messengers that mediate intracellular calcium mobilization, thereby regulating critical physiological processes such as lymphocyte proliferation, insulin secretion, and T cell activation. Notably, CD38 forms an immunoregulatory network with other metabolic enzymes: by degrading ATP and NAD⁺ to generate adenosine, it contributes to the formation of immunosuppressive microenvironments, highlighting its multifaceted role in immune regulation.
2. Research Frontiers
2.1 Structural and Functional Traits of the CD38 Molecule
Beyond its enzymatic activities, CD38’s structural features support its diverse functions. The extracellular domain, responsible for substrate binding and catalysis, enables interactions with multiple ligands and signaling molecules. Its ability to form homodimers or oligomers on the cell surface may enhance enzymatic efficiency and signal transduction. Importantly, CD38’s dual role in nucleotide metabolism and immune modulation positions it as a critical node linking metabolic homeostasis to immune cell function—making it a promising target for therapeutic intervention in diseases driven by immune dysregulation and metabolic imbalance.
2.2 CD38 Expression Profiles in Physiological and Pathological States
Physiological Expression: Under normal conditions, CD38 is predominantly expressed in bone marrow and lymphoid tissues, with minimal to no expression on hematopoietic stem cells. This restricted distribution provides a safety margin for targeted therapies, as it minimizes potential damage to healthy stem cell populations.
Pathological Expression: In hematologic malignancies, CD38 exhibits high expression in most cases of multiple myeloma (MM) and AIDS-related lymphomas. It is also significantly expressed in acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and chronic lymphocytic leukemia (CLL). Beyond hematopoietic tumors, abnormal CD38 expression is associated with pathological processes in solid organs, including heart disease, autoimmune disorders (e.g., rheumatoid arthritis), and metabolic diseases—expanding its potential as a diagnostic and therapeutic target.
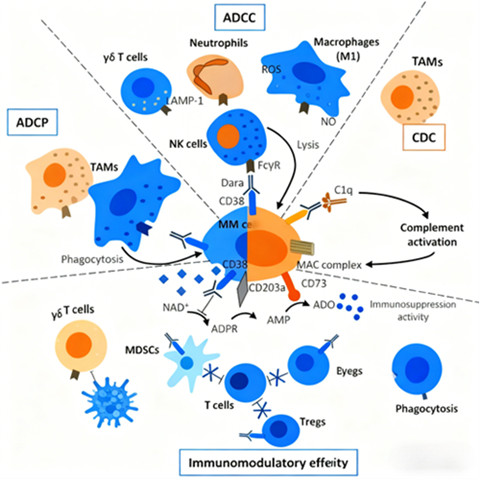
2.3 Mechanisms of Action of CD38 Antibody Drugs
CD38-targeted antibodies exert antitumor effects through a synergistic multi-mechanistic approach:
Fc-Dependent Cytotoxicity: Antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) directly mediate tumor cell lysis. ADCC involves the activation of immune effector cells (e.g., natural killer cells, macrophages) via Fc receptor binding, while ADCP promotes phagocytosis of tumor cells by macrophages. CDC activates the complement cascade, leading to the formation of the membrane attack complex (MAC) and subsequent tumor cell death.
Immune Modulation: CD38 antibodies inhibit the ectoenzyme activity of CD38, reducing the production of immunosuppressive adenosine. They also eliminate CD38-positive immunosuppressive cells (e.g., regulatory T cells, myeloid-derived suppressor cells), thereby restoring the function of effector T cells and enhancing antitumor immune responses. This dual action—direct tumor cell killing and immune microenvironment remodeling—underpins the clinical efficacy of CD38 antibodies.
2.4 Clinical Value of CD38-Targeted Therapy
CD38-targeted therapy has emerged as a transformative approach for hematologic malignancies:
Broad Applicability: It demonstrates therapeutic potential across a range of blood cancers, from pediatric to adult patients, including AML, T-cell ALL, and MM.
Overcoming Resistance: Clinical data show that CD38 antibody drugs effectively overcome resistance to conventional chemotherapies and other targeted agents, providing new treatment options for relapsed/refractory patients with limited therapeutic alternatives.
Combination Synergy: CD38-targeted therapy exhibits promising synergistic effects when combined with other immunotherapies (e.g., CAR-T cells, immune checkpoint inhibitors), chemotherapy, or proteasome inhibitors—enhancing overall treatment efficacy and extending patient survival.
2.5 Development History and Technical Features of CD38 Antibody Drugs
The development of CD38 antibodies represents a trajectory of technological innovation:
Antibody Engineering: Early development leveraged genetically engineered mouse platforms to generate fully humanized antibodies, reducing immunogenicity in clinical applications. Rigorous epitope screening and functional validation were conducted to identify candidates with high binding affinity to CD38 and potent effector functions (e.g., ADCC, CDC).
Preclinical Validation: Candidate antibodies were evaluated in various xenograft models to confirm their ability to inhibit tumor growth and prolong survival. Studies focused on optimizing antibody properties such as Fc region modifications to enhance effector function and half-life, ensuring robust clinical performance.
2.6 Future Directions for CD38-Targeted Therapy
As research into CD38 deepens, several promising directions are emerging:
Novel Therapeutic Formats: Development of CD38-based cell therapies (e.g., CD38-targeted CAR-T cells) and next-generation bispecific antibodies (e.g., CD38/CD3 bispecific antibodies that redirect T cells to tumor cells) to enhance targeting precision and efficacy.
Expanded Indications: Exploration of CD38-targeted therapy in solid tumors, where abnormal CD38 expression is associated with disease progression.
Optimal Combination Strategies: Investigation of synergistic combinations with conventional chemotherapy, immune checkpoint inhibitors, and other targeted agents to improve treatment outcomes and overcome resistance.
Biomarker Development: Identification of predictive biomarkers to select patients most likely to respond to CD38-targeted therapy, enabling personalized treatment approaches.
3. Research Significance
CD38-targeted therapy addresses a critical unmet need in hematologic malignancy treatment, particularly for relapsed/refractory patients with limited options. By leveraging CD38’s unique structural and functional properties, antibodies targeting this molecule deliver synergistic antitumor effects through direct cell killing and immune modulation. Beyond clinical applications, research on CD38 has deepened our understanding of the interplay between nucleotide metabolism and immune regulation, opening new avenues for investigating immune dysregulation in other diseases. Additionally, CD38’s diverse expression profile across malignancies and non-malignant conditions positions it as a versatile diagnostic marker, supporting early disease detection and prognosis assessment.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
CD38’s biological effects are mediated through two core pathways:
Nucleotide Metabolism: As a bifunctional ectoenzyme, CD38 catalyzes the conversion of NAD⁺ to cADPR and adenosine, regulating intracellular calcium signaling and immune cell function.
Immune Regulation: CD38 contributes to immunosuppressive microenvironment formation by generating adenosine and interacting with immunosuppressive cell populations. CD38 antibodies disrupt these pathways, restoring immune homeostasis and promoting antitumor immunity.
4.2 Research Methods
Key research methods for studying CD38 include:
Expression Analysis: Immunohistochemistry (IHC), flow cytometry, and Western blotting to detect CD38 expression in tissues and cells.
Functional Assays: Enzymatic activity assays to measure CD38’s cyclase and hydrolase functions; ADCC, ADCP, and CDC assays to evaluate antibody-mediated cytotoxicity.
In Vitro and In Vivo Models: Cell line-based studies to assess tumor cell killing; xenograft mouse models to validate in vivo efficacy of CD38-targeted therapies.
Clinical Research: Clinical trials to evaluate safety, efficacy, and pharmacodynamics of CD38 antibodies in patients with hematologic malignancies.
4.3 Product Applications
ANT BIO PTE. LTD.’s CD38 antibodies, led by the STARTER brand’s "S-RMab® CD38 Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2038), are essential tools for research and clinical diagnostics:
Multiple Myeloma Diagnosis and Monitoring: As a key diagnostic marker for MM, the antibody enables tumor cell detection and quantitative analysis, supporting patient stratification and treatment response assessment for CD38-targeted therapies (e.g., daratumumab).
Plasma Cell Disorder Research: Aids in the auxiliary diagnosis and differentiation of Waldenström macroglobulinemia, plasmacytoma, and other plasma cell disorders.
Lymphocyte Activation and Function Studies: Facilitates investigation of CD38 expression on activated T cells, B cells, and NK cells, and its role in cell signaling and metabolic regulation.
Metabolic-Immune Crosstalk Research: Supports exploration of CD38’s novel functions in NAD⁺ metabolism, calcium signaling, and immunometabolism—key areas of interest in immune-oncology.
The S0B2038 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant rabbit monoclonal platform and validated for IHC, offers exceptional advantages: high specificity with clear membrane/cytoplasmic localization (ensuring reliable detection in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community with high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in oncology, immunology, and metabolic research. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through cutting-edge life science research and clinical translation.
6. Related Product List
| Catalog No. | Product Name | Host |
| S0B2038 | S-RMab® CD38 Recombinant Rabbit mAb (SDT-031-45-2) | Rabbit |
| S0B1635 | Alexa Fluor® 488 Mouse Anti-Human CD38 Antibody (S-R507-1) | Mouse |
| S0B1069 | Rat anti-Mouse CD38 Antibody (S-R549) | Rat |
| S0B5034 | FITC Rat Anti-Mouse CD38 Antibody (S-R549) | Rat |
| S0B5132 | PE-Cy7 Rat Anti-Mouse CD38 Antibody (S-R549) | Rat |
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026
- Eppendorf Collaborates with Dubai Police to Automate Forensics Laboratories 2/9/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/9/2026
- Reshaping Hematologic Malignancy Treatment: Multi-Mechanism Synergy of CD38 Anti 2/5/2026
- Optimizing In Vitro T Cell Activation and Expansion: The Pivotal Role of CD3 Eps 2/4/2026
- Eppendorf & Science Prize for Neurobiology 2026: Call for Entries! 2/4/2026


