Product Overview
The Ochratoxin A Rapid Test Kit is a professional-grade rapid detection solution for Ochratoxin A contamination in food and feed matrices.
It is specifically developed for high-throughput, on-site screening of grains commonly used in Europe and Africa, including corn, wheat, maize, rice, and peanuts, as well as animal feed and selected herbal materials.
The test kit is:
• Fully EU MRL compliant
• Validated against LC-MS/MS reference methods
• Suitable for both qualitative and quantitative analysis
• Proven through long-term, large-scale global use
⸻
Key Advantages — What Makes This Kit Different
✅ Rapid On-Site, Large-Batch Screening
• Results in 5–10 minutes
• Ideal for grain silos, feed mills, warehouses, and ports
• Supports high-frequency decision making without lab delays
⸻
✅ EU Standard & LC-MS/MS Equivalent Accuracy
• Fully aligned with EU Maximum Residue Limits (MRL)
• Strong correlation with LC-MS/MS laboratory data
• Reliable for regulatory screening and export compliance
⸻
✅ Quantitative & Qualitative Detection
• Enables risk grading, not just pass/fail
• Suitable for field screening + laboratory confirmation
• Supports quality control across the supply chain
⸻
✅ Cost-Effective & Easy to Use
• No complex instruments required
• Minimal training needed
• Significantly reduces routine LC-MS/MS testing costs
⸻
Proven Beyond the Laboratory — Trusted by the Real World
Unlike many suppliers whose Ochratoxin A test kits are only short-term laboratory-validated, our Ochratoxin A Rapid Test Kit has been proven through tens of millions of real-world tests across global grain, feed, and food safety systems.
With over 20 years of dedicated rapid test manufacturing experience, our solutions have been selected for national food safety programs and major international sporting events, including the Olympic Games and Asian Games, where accuracy, stability, and zero tolerance for error are mandatory.
This depth of long-term, high-volume validation clearly distinguishes us from ordinary manufacturers.
Our reliability is not a marketing promise — it is continuously verified under the most demanding real-world conditions worldwide.
Typical Applications
• Grain & Cereal Screening
Corn, wheat, maize, rice, peanuts
• Animal Feed & Raw Material Intake Inspection
• Export & Import Quality Control
• Laboratories & Third-Party Testing Centers
• Government & Regulatory Monitoring Programs
⸻
Technical Specifications
Item Description
Target Ochratoxin A
Detection Type Qualitative & Quantitative
Detection Time 5–10 minutes
Accuracy Equivalent to LC-MS/MS
Compliance EU MRL, CE, ISO
Sample Types Grains, feed, herbs
Storage 4–30°C
Shelf Life 15 months
STORAGE AND STABILITY
Store at room temperature(4-30℃).
DO NOT FREEZE or use beyond the expiration date.
The shelf life is 15 months.
KIT CONTENTS
-SmarKIT Ochratoxin A
Rapid Test (40/50 tests /kit)
-Plastic dropper (40/50 pieces /kit)
-PBST Buffer (1 bottle/kit, 30ml)
-Buffer A (1 bottle/kit, 15ml)
-Packing Insert (1 set /kit)
TEST PROCEDURE
Read the entire procedure carefully before performing any test.
Keep the test kit and reagent at room temperature(20℃~30℃) before testing.
1. Prepare samples according to chapter 7 (Sample preparations).
2. Remove the Ochratoxin A
Rapid Test from sealed pouch.
3. Hold the dropper vertically and transfer 3 full drops(around 100µL) of solution to the specimen well (S) and then start the timer.
4. Wait for red bands to appear. The result should be read in approximately 3-5 minutes. Do not interpret results after 5 minutes.
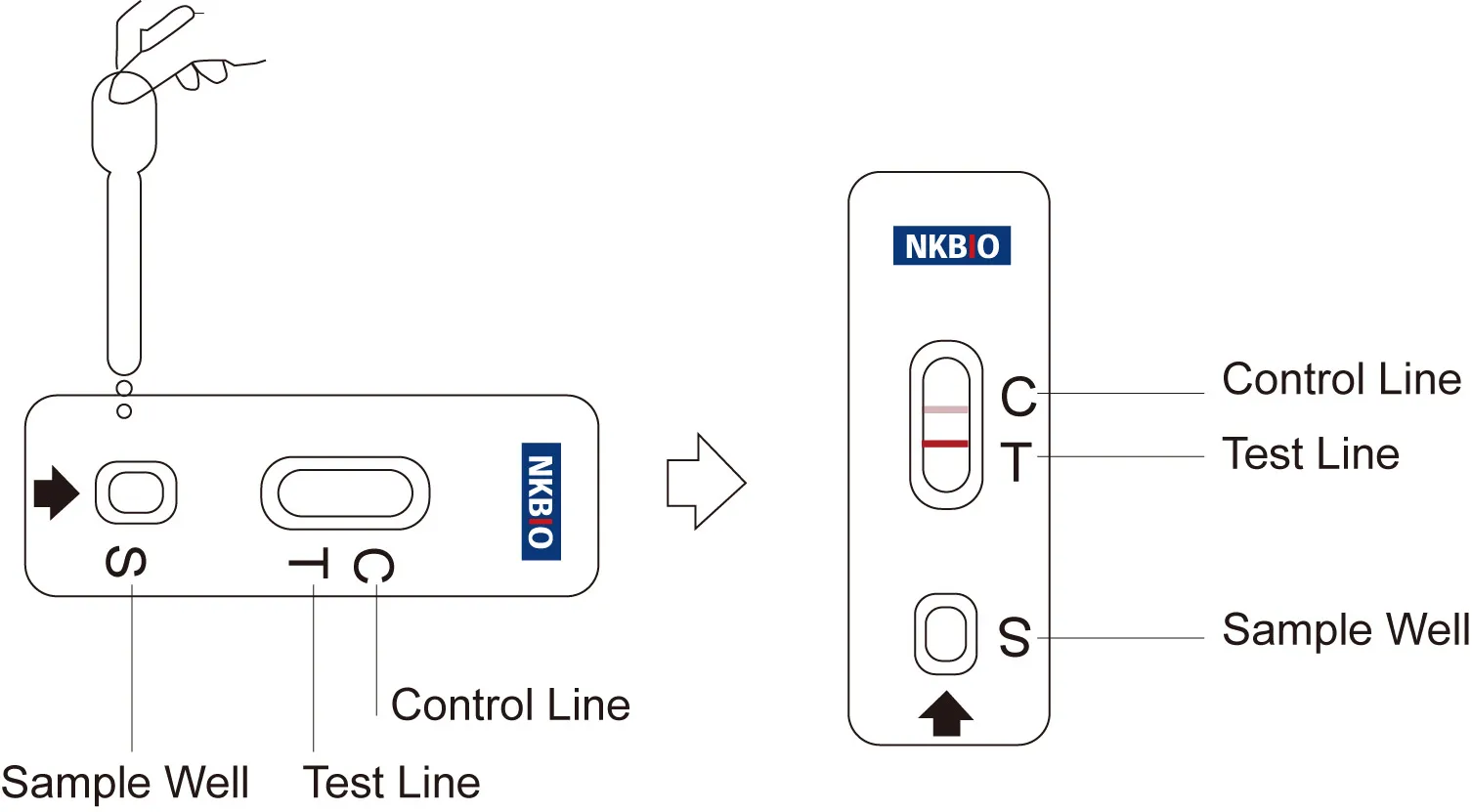 Reading results
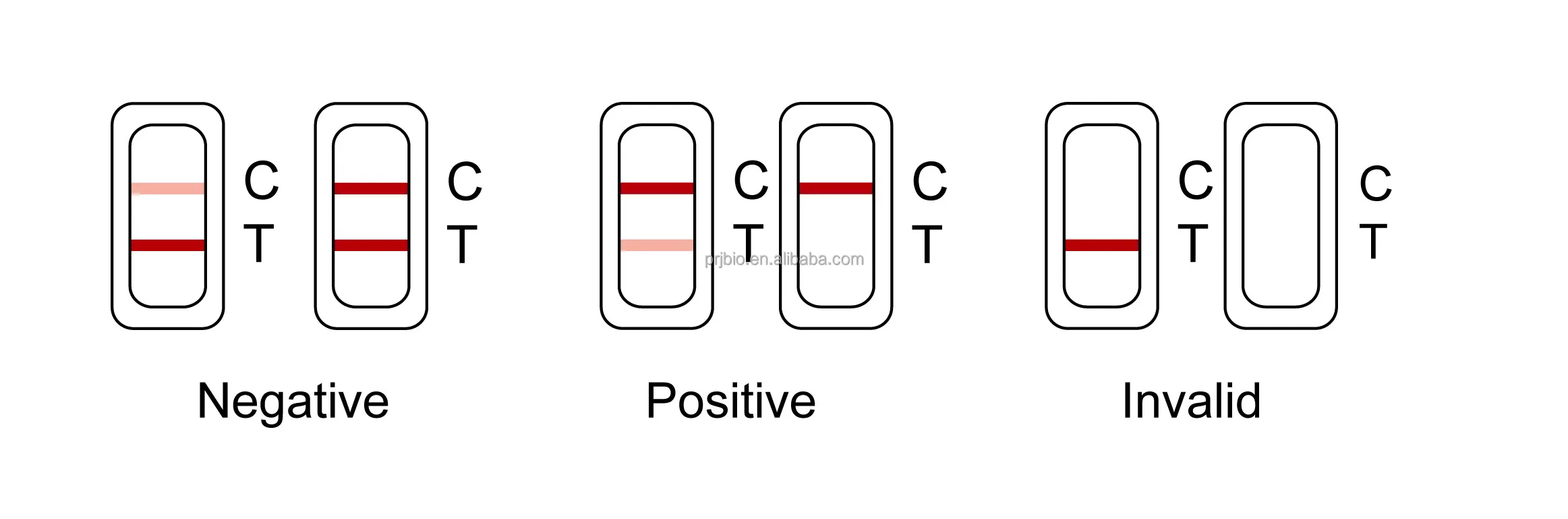
Negative:
The Test Line (T) is the same as or darker than the Control Line (C). It is negative.
POSITIVE:
The Test Line (T) is lighter than the Control Line (C), or there is no Test Line. It is positive.
INVALID: Reference Line fails to appear.
Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for an invalid result.
Reading results
Negative:
The Test Line (T) is the same as or darker than the Control Line (C). It is negative.
POSITIVE:
The Test Line (T) is lighter than the Control Line (C), or there is no Test Line. It is positive.
INVALID: Reference Line fails to appear.
Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for an invalid result.
Review the procedure and repeat the test with a new test kit. Stop using the test kit immediately if the problem is not solved and contact your local distributor.
bio-equip.cn
Established in 2005, NKBIO Group Co., Ltd. is a national high-tech enterprise dedicated in developing, manufacturing and marketing Rapid Test Kits in Food and Feed Analysis, Animal Disease as well as Clinical Diagnostics.
The Rapid Tests can detect the animal disease, residues of antibiotics, mycotoxins, veterinary drugs, pesticides, microorganism, heavy metal, illegal additives and toxic chemicals in all kind of foods and feeds. It is an accurate, cost-effective method as a screening solution, useful for both labs and field testing, can also be applied for the quality control “From farm to fork”.
NKBIO have three Advanced Third Party Independent laboratories to guarantee, further confirm the results from the preliminary Positive & Negative Samples, have capability to issue formally, official testing reports which acknowledged by both government authority and academic institutions.
With Rapid Testing Branch, Software Engineering & Equipment Branch and Service of complete solution Branch, we have successfully built Food & Feed Quality Management Traceability System for regulation of Government Authority and Enterprises.
NKBIO values the innovation, have widely technological and scientific research cooperation with national top-level universities and scientific research institutes, like Zhejiang University, Jinan University, South China Agricultural University, Academy of Agricultural Sciences. We have published many of technical literatures, got hundreds of prizes & certificates, and also undertaken making rules and regulations for Food and Feed testing standards for government authority.
For more information please visit:
www.nkbiotech.com
http://www.foodrapidtest.com
E -mail: sale@nkbiotech.com
bellafu88@yahoo.com
WhatsApp:0086-151-5809-6930