Sample Collection, Processing, and Storage Methods1. Serum: Use pyrogen- and endotoxin-free tubes. Avoid any cell stimulation during the procedure. After blood collection, centrifuge at 3000 rpm for 10 minutes to quickly and carefully separate the serum from the red blood cells. 2. Plasma: Anticoagulate with EDTA, citrate, or heparin. Centrifuge at 3000 rpm for 30 minutes and remove the supernatant. 3. Cell Supernatant: Centrifuge at 3000 rpm for 10 minutes to remove particles and aggregates. 4. Tissue Homogenate: Add an appropriate amount of saline to the tissue and mash. Centrifuge at 3000 rpm for 10 minutes and remove the supernatant. 5. Storage: If samples are not tested promptly after collection, aliquot them into single-use aliquots and freeze at -20°C to avoid repeated freezing and thawing. Thaw at room temperature and ensure the sample is evenly and fully thawed.
Reagent PreparationDilution of 20× Wash Buffer: Dilute distilled water 1:20, i.e., add 1 part 20× Wash Buffer to 19 parts distilled water. Plate Washing Method1. Manual Plate Washing: Shake off all liquid in the wells, fill each well with wash buffer, let stand for 1 minute, shake off all liquid in the wells, and pat dry on absorbent paper. Wash the plate 5 times in this manner. 2.Automatic plate washer: Add 350 μL of wash solution to each well, soak for 1 minute, and wash the plate 5 times.
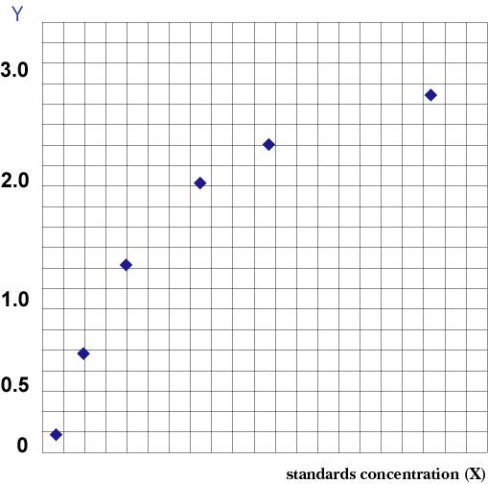
Operational Steps 1. Remove the desired strips from the aluminum foil bag after equilibration at room temperature for 20 minutes. Seal the remaining strips in a ziplock bag and return them to 4°C. 2. Set up standard wells and sample wells. Add 50 μL of a standard of varying concentration to each standard well. 3. First, add 10 μL of the sample to be tested to the sample well, followed by 40 μL of sample diluent. Do not add anything to the blank well. 4. Add 100 μL of horseradish peroxidase (HRP)-labeled detection antibody to each standard and sample well, except for the blank well. Seal the wells with sealing film and incubate at 37°C in a waterbath or incubator for 60 min. 5. Discard the liquid, pat dry on absorbent paper, then fill each well with wash buffer. Let stand for 1 min, discard the wash buffer, and pat dry on absorbent paper. Repeat this process five times (a microplate washer can also be used). 6. Add 50 μL each of substrates A and B to each well and incubate at 37°C in the dark for 15 min. 7. Add 50 μL of stop solution to each well and measure the OD value of each well at 450 nm within 15 min. Result Evaluation Draw a standard curve: In an Excel worksheet, use the standard concentration as the horizontal axis and the corresponding OD value as the vertical axis to draw a linear regression curve for the standard. Calculate the concentration of each sample according to the curve equation.
 |