Sample handling and requirements1.Serum:The whole blood sample collected in the serum separation tube is placed at room temperature for 2 hours or 4 ℃ overnight, and then centrifuged at 1000 × g for 20 minutes. The supernatant can be taken, or the supernatant can be stored at-20 ℃ or-80 ℃, but repeated freezing and thawing should be avoided. 2.Plasma:Specimens were collected using either EDTA or heparin as anticoagulant and subjected to the specimens within 30 minutes after collection at 2-8 °C 1000 × g centrifuge for 15 minutes, take the supernatant for detection, or store the supernatant at-20 ℃ or-80 ℃, but avoid repeated freezing and thawing. 3.Tissue homogenate:The tissues were washed with pre-cooled PBS (0.01 M, pH = 7.4) to remove residual blood (lysed red blood cells in the homogenate would affect the measurement results), and the tissues were sheared after weighing. Combine the chopped tissue with the corresponding volume of PBS (generally according to the weight-to-volume ratio of 1: 9, for example, 1g of tissue sample corresponds to 9mL of PBS. The specific volume can be appropriately adjusted according to the needs of the experiment and recorded. It is recommended to add protease inhibitor to PBS) add to a glass homogenizer and grind thoroughly on ice. For further lysis of tissue cells, the homogenate can be sonicated, or freeze-thawed repeatedly. Finally, the homogenate was centrifuged at 5000 × g for 5-10 minutes, and the supernatant was taken for detection. 4.Cell culture supernatant or other biological specimen:Please centrifuge at 1000 × g for 20 minutes, take the supernatant for detection, or store the supernatant at-20 ℃ or-80 ℃, but avoid repeated freezing and thawing. Note: Hemolysis of the specimen will affect the final test result, so hemolyzed specimens should not be tested.
Reagent Preparation The kit should be removed from the refrigerated environment and equilibrated at room temperature before use. Dilution of 20 × wash buffer: Distilled water was diluted 1:20, i.e. 1 part of 20 × wash buffer plus 19 parts of distilled water. Operation steps1. Take out the required slats from the aluminum foil bag after equilibration at room temperature for 20 minutes, and seal the remaining slats with a ziplock bag and put them back to 4 °C. 2. Set up standard wells and sample wells, and add 50μL of different concentrations of standards to each standard well; 3. Add 50μL of the sample to be tested to the sample well; Blank holes are not added. 4. Add horseradish peroxidase (HRP)-labeled detection antibody to each well of the standard well and sample well except for the blank well 100 μL, seal the reaction wells with a plate sealing membrane, and incubate for 60 minutes in a 37 °C water bath or incubator. 5. Discard the liquid, pat dry on absorbent paper, fill each well with washing liquid (350μL), let it stand for 1min, throw away the washing liquid, pat dry on absorbent paper, and repeat washing the plate 5 times (you can also use a plate washing machine to wash the plate). 6. Add 50 μL of each substrate A and B to each well, and incubate at 37 °C in the dark for 15 minutes. 7. Add 50 μL of stop solution to each well, and measure the OD value of each well at a wavelength of 450nm within 15 minutes. Calculation of experimental resultsTaking the OD value of the measured standard product as the abscissa and the concentration value of the standard product as the ordinate, draw the standard curve on coordinate paper or with relevant software, and get the linear regression equation. Substitute the OD value of the sample into the equation to calculate the concentration of the sample.
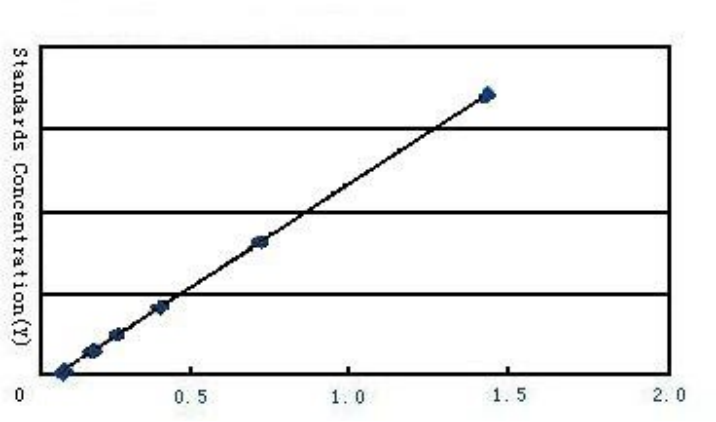 Standard curve Standard curve
Note: Repeatability: The intra-plate coefficient of variation is less than 10% and the inter-plate coefficient of variation is less than 15%.
| 




 Standard curve
Standard curve