Reagents:
Molecular Biology
Biochemistry
Cell Biology
ELISA / Diagnostic Kits
Antibody
Serum/Medium
Other Reagents
- Human PAI-1 ELISA Kit

- Product Detail
- Company Profile
- Preparation of HRP Detection Antibody Working Solution: 15 minutes before use, centrifuge 100µl of concentrated HRP Detection Antibody at 1000µg for 1 minute. Dilute the 100µl concentrated HRP Detection Antibody to a 1µl working concentration with universal diluent (e.g., 10µl concentrate + 990µl universal diluent). Prepare immediately before use. Preparation of 1× Wash Buffer: Dissolve 10 mL of 20× Wash Buffer in 190 mL of distilled water. (Centrated Wash Buffer removed from the refrigerator may crystallize, which is normal. Allow to stand at room temperature until the crystals have completely dissolved before preparing the 1× Wash Buffer.) Required Instruments: 1. Microplate reader (450 nm) 2. High-precision pipettes and tips: 5-10 μL, 5-50 μL, 20-200 μL, 200-1000 μL 3. 37°C incubator 4. Distilled or deionized water Sample Handling and Requirements: 1. The detection range of the kit is not equivalent to the concentration range of the analyte in the sample. It is recommended to estimate the concentration of the analyte in the sample based on relevant literature and to conduct preliminary experiments to determine the actual concentration in the sample. If the concentration of the analyte in the sample is too high or too low, please dilute or concentrate the sample appropriately.
2. If the sample being tested is not among the sample types listed in the instructions, it is recommended to conduct a preliminary experiment to verify the validity of the test.
3. Serum: Collect whole blood in a serum separator tube and place it at room temperature for 2 hours or at 2-8°C overnight. Then centrifuge it at 1000×g for 20 minutes and remove the supernatant. Alternatively, store the supernatant at -20°C or -80°C, but avoid repeated freezing and thawing.
4. Plasma: Collect the sample using EDTA or heparin as an anticoagulant. Within 30 minutes of collection, centrifuge it at 1000×g for 15 minutes at 2-8°C. Remove the supernatant and test it. Alternatively, store the supernatant at -20°C or -80°C, but avoid repeated freezing and thawing. 5. Tissue Homogenization: Rinse the tissue with pre-chilled PBS (0.01M, pH 7.4) to remove any residual blood (lysed red blood cells in the homogenate will affect test results). Weigh the tissue and mince it. Add the minced tissue to the appropriate volume of PBS (generally a 1:9 weight-to-volume ratio, e.g., 1g of tissue sample to 9mL of PBS. The specific volume can be adjusted according to experimental needs and recorded. It is recommended to add protease inhibitors to the PBS) in a glass homogenizer and grind thoroughly on ice or in a homogenizer. To further lyse tissue cells, the homogenate can be sonicated or repeatedly frozen and thawed. Finally, centrifuge the homogenate at 5000×g for 5-10 minutes and remove the supernatant for analysis. 6. Cell Culture Supernatant: Centrifuge at 1000×g for 20 minutes. Remove the supernatant for analysis or store at -20°C or -80°C, avoiding repeated freezing and thawing. 7. Cell Lysis Buffer: Gently wash adherent cells with pre-chilled PBS, then trypsinize and collect the cells by centrifugation at 1000 × g for 5 minutes. Suspension cells can be collected directly by centrifugation. Wash the collected cells three times with pre-chilled PBS and resuspend them in 150-200 μL of PBS per 1 × 10^6 cells (it is recommended to add protease inhibitors to the PBS; if the cell count is very low, the PBS volume can be reduced appropriately). Disrupt the cells by repeated freeze-thaw cycles or sonication. Centrifuge the extract at 1500 × g for 10 minutes at 2-8°C, and remove the supernatant for analysis. 8. Other Biological Specimens: Centrifuge at 1000 × g for 20 minutes, and collect the supernatant for analysis. 9. Sample Appearance: The sample should be clear and transparent, and any suspended matter should be removed by centrifugation. 10. Sample Storage: Samples collected for testing within one week can be stored at 4°C. If testing cannot be performed promptly, aliquot the sample into single-use portions and store at -20°C (for testing within one month) or -80°C (for testing within six months). Avoid repeated freezing and thawing. Hemolysis of the sample can affect the final test results, so hemolyzed samples are not suitable for this test. Sample Dilution Protocol: Please estimate the concentration range of your sample in advance. If your sample requires dilution, the following dilution protocol is recommended: 100-fold dilution: One-step dilution. Dispense 5 μL of sample into 495 μL of universal diluent and perform a 100-fold dilution. 1000-fold dilution: Two-step dilution. Take 5 μL of sample into 95 μL universal diluent and make a 20-fold dilution. Then take 5 μL of the 20-fold diluted sample into 245 μL universal diluent and make a 50-fold dilution, for a total dilution of 1000 times. Dilution 100,000 times: three-step dilution. Transfer 5 μL of sample to 195 μL of universal diluent and dilute 40-fold. Then, transfer 5 μL of the 40-fold diluted sample to 245 μL of universal diluent and dilute 50-fold. Finally, transfer 5 μL of the 2,000-fold diluted sample to 245 μL of universal diluent and dilute 50-fold, for a total dilution of 100,000-fold. For each dilution step, transfer at least 3 μL of solution, and the dilution factor should not exceed 100-fold. Mix thoroughly during each dilution step to avoid foaming. Procedure: 1. Remove the desired strips from the aluminum foil bag after equilibration at room temperature for 10 minutes. Seal the remaining strips in a ziplock bag and return them to 4°C. 2. Sample Addition: Add 100 μL of sample or standard of varying concentrations to the corresponding wells. Add 100 μL of universal diluent to the blank wells. Cover with a film sealer and incubate at 37°C for 60 minutes. (Recommendation: Dilute the test samples to a minimum of 1-fold with universal diluent before adding them to the plate. This will minimize matrix effects on the test results. The sample concentration should be multiplied by the dilution factor when calculating the final sample concentration. It is recommended to run replicates for all test samples and standards.) 3. Plate Wash: Discard the remaining liquid. Add 300 μL of 1x wash buffer to each well. Let stand for 1 minute, then shake off the wash buffer and pat dry on absorbent paper. Repeat this process three times (a plate washer can also be used). 4. Add HRP Detection Antibody: After washing, add 100 μL of HRP Detection Antibody working solution directly to each well. Cover with a film sealer and incubate at 37°C for 60 minutes. 5. Wash: Discard the liquid and wash the plate five times as in step 3. 6. Add substrate: Add 90 μL of substrate (TMB) to each well, cover with sealing film, and incubate at 37°C in the dark for 15 minutes. 7. Add stop solution: Remove the plate and add 50 μL of stop solution directly to each well. Immediately measure the OD value of each well at 450 nm. Calculation of experimental results: 1. Calculate the average OD value of the standard and sample replicates and subtract the OD value of the blank well as a correction factor. Plot a four-parameter logistic function standard curve on double-logarithmic graph paper with concentration as the horizontal axis and OD value as the vertical axis. 2. If the sample OD value is higher than the upper limit of the standard curve, dilute the sample and retest it. Multiply the sample concentration by the corresponding dilution factor.
The following data and curves are for reference only. Experimenters should establish a standard curve based on their own experiments.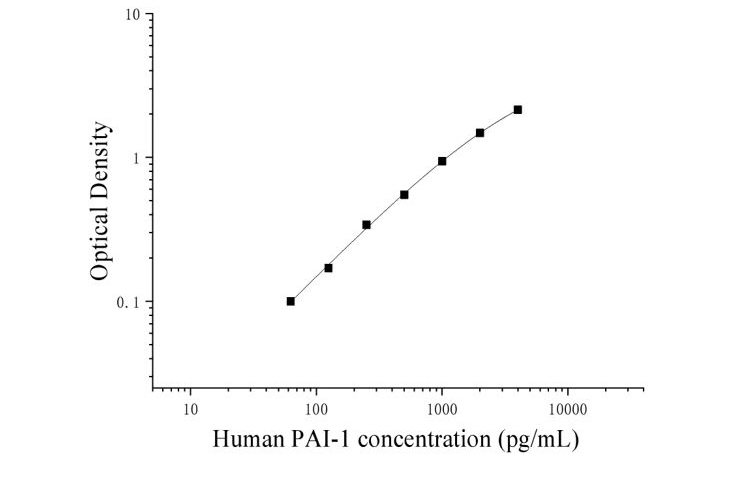
Note: This graph is for reference only. Sample content should be calculated based on the standard curve drawn from each experimental data.
Kit Performance:
1. Repeatability: Intra-assay coefficient of variation (CV) <10%, inter-assay coefficient of variation <10%.
2. Recovery: Human PAI-1 was spiked into healthy human plasma, tissue homogenate, and cell lysate at three different concentrations, and the recovery was calculated.Sample type
Range (%)
Average recovery rate (%)
Plasma(n=8)
84-101
96
Tissue homogenate (n=8)
92-105
102
Cell lysate (n=8)
Dilution ratio
Recovery rate (%)
Plasma
Tissue homogenate
Cell lysate
1:2
range
84-95
88-96
90-110
Average recovery rate
91
93
96
1:4
range
89-103
87-108
105-115
Average recovery rate
94
98
108
Problem Analysis:If the experimental effect is not good, please take a photo of the color development result in time, save the experimental data, keep the used plates and unused reagents, and then contact our technical support to solve the problem for you. You can also refer to the following information:
Problem description Possible causes Corresponding countermeasures Poor linearity of calibration curve Incorrect dilution of standard Ensure that the standards are dissolved and diluted according to the recommended method Inaccurate pipetting Regularly calibrate the pipette and check the seal of the pipette tip Evaporation of reaction solution Seal the ELISA plate with sealing film Incomplete plate washing Enough washing times and adding enough washing solution Foreign matter on the bottom of the well Clean the bottom of the plate before reading Weak or colorless color Insufficient incubation time Ensure the incubation time Incorrect incubation temperature Insufficient reagent volume Check pipette and follow protocol carefully Incorrect dilution Check reagent dilution procedure Enzyme conjugate inactivated Mix enzyme conjugate and substrate and check by colorimetric reaction Theory This kit uses a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Samples, standards, and HRP-labeled detection antibodies are sequentially added to microwells pre-coated with human plasminogen activator inhibitor 1 (PAI-1) capture antibodies. After incubation and washing, the sample is developed using the substrate TMB. TMB converts to blue under the catalysis of HRP peroxidase and to yellow under the action of acid. The intensity of the color is positively correlated with the amount of human plasminogen activator inhibitor 1 (PAI-1) in the sample. The absorbance (OD) is measured at 450 nm using a microplate reader, and the sample concentration is calculated. Sensitivity: 24.93 pg/mL. Specificity: Detects human PAI-1 in samples with no significant cross-reactivity with its analogs. Source Human Synonym Human Plasminogen Activator Inhibitor 1 ELISA Kit Detection Type Double antibody sandwich method Composition Name 96-hole configuration Remarks Pre-coated 96-well enzyme plate 8 wells×12 strips None Standard product 2 pieces Dilute according to instructionsUniversal diluent 2×20mL120uLDilution according to the instructions20×Washing liquid 2×10mLDilute according to instructionsSubstrate (TMB) 10mLnoneStop solution 6mLNoneSealing film 4 picturesNoneInstructions 1 copyNoneBackground PAI-1, also known as endothelial prothrombin activator inhibitor or serum protein E1, is a serine protease inhibitor (serpin). Its primary function is to inhibit urokinase plasmakinase activator (uPA), an enzyme responsible for cleaving plasmakinogen to form plasmakin. Plasmin, either by itself or in conjunction with matrix metalloproteinases, mediates the degradation of the extracellular matrix. In this context, PAI-1 inhibits uPA by binding to its active site, preventing plasmakin formation. Additional inhibition occurs through PAI-1 binding to the uPA/uPA receptor complex, leading to its degradation. Therefore, PAI inhibits the serine proteases tPA and uPA/urokinase, thereby acting as an inhibitor of fibrinolysis, the physiological process that degrades blood clots. Furthermore, PAI-1 inhibits the activity of matrix metalloproteinases, which play a key role in the invasion of malignant cells through the basal lamina. General Notes 1. Strictly adhere to the specified incubation time and temperature to ensure accurate results. All reagents must be at room temperature (20-25°C) before use. Refrigerate reagents immediately after use.
2. Improper plate washing may result in inaccurate results. Ensure that all liquid in the wells is aspirated thoroughly before adding substrate. Do not allow the wells to dry out during incubation.
3. Remove any residual liquid and fingerprints from the bottom of the plate, as this will affect the OD value.
4. The substrate developer solution should be colorless or very light in color. Do not use substrate solution that has turned blue.
5. Avoid cross-contamination of reagents and specimens to prevent erroneous results.
6. Avoid direct exposure to strong light during storage and incubation.
7. Do not expose any reagents to bleaching solvents or the strong fumes emitted by bleaching solvents. Any bleaching agent will destroy the biological activity of the reagents in the kit.
8. Do not use expired products, and do not mix components with different product numbers and batches.
9. Recombinant proteins from sources other than the kit may not be compatible with the antibodies in this kit and will not be recognized.
10. If there is a possibility of disease transmission, all samples should be managed properly and samples and testing devices should be handled according to prescribed procedures.Storage Temp. If the unopened kit is stored at 4°C, the shelf life is 6 months. Test Range 62.5-4000pg/mL Applications Plasma, tissue homogenate, cell lysate, cell culture supernatant and other biological fluids bio-equip.cn-
AntBio is a biotechnology group company dedicated to serving life sciences, aiming to help scientists accelerate research and improve work efficiency. AntBio provides comprehensive and high-quality reagent tools for basic research, drug development, and diagnosis, including research grade antibodies, proteins, biochemical reagents, and assay kits. These research tools are widely used in different segments of life science research. The group company currently consists of three brands, Absin, Starter-Bio and UA-Bio.
Request Information
Other Products Related Products
Recently viewed products - SiteMap
Product Specification
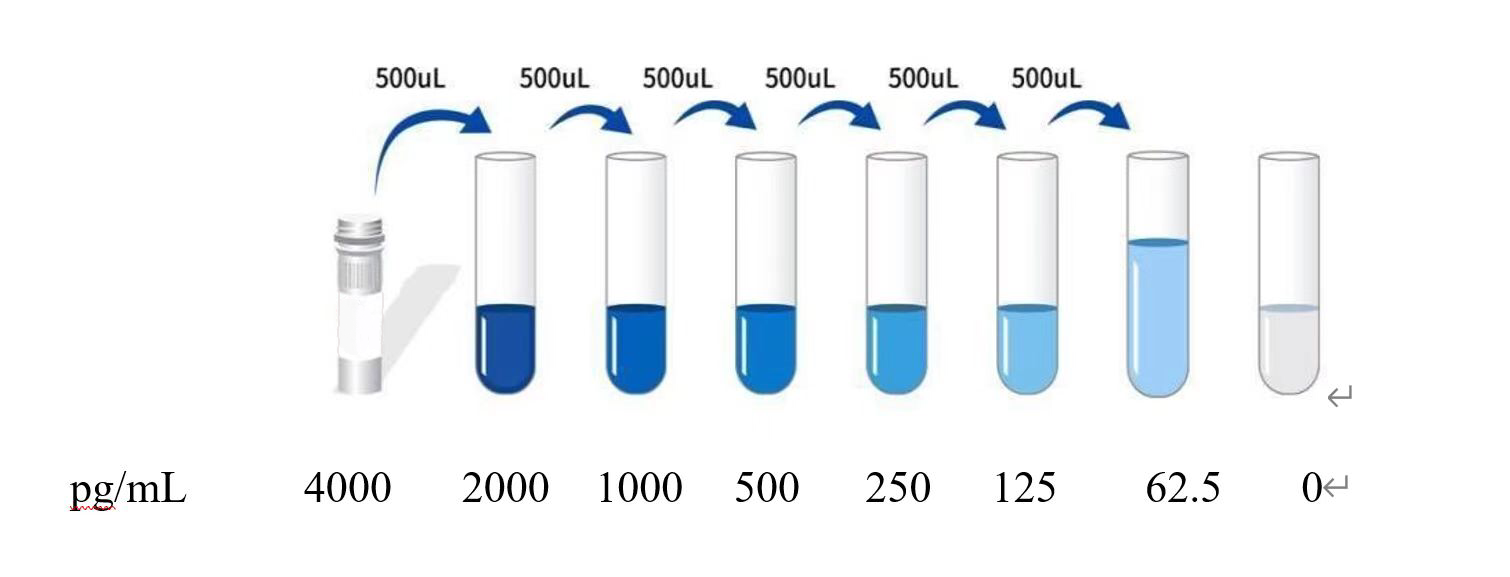
| Usage | Preparation before testing:1. Remove the test kit from the refrigerator 10 minutes in advance and equilibrate to room temperature.2. Preparation of Gradient Standard Working Solution: Add 1 mL of Universal Diluent to the lyophilized standard. Let stand for 15 minutes to completely dissolve, then gently mix (concentration is 4000 pg/mL). Then, dilute to the following concentrations: 4000 pg/mL, 2000 pg/mL, 1000 pg/mL, 500 pg/mL, 250 pg/mL, 125 pg/mL, 62.5 pg/mL, and 0 pg/mL. Serial Dilution Method: Take 7 EP tubes and add 500 μL of Universal Diluent to each tube. Pipette 500 μL of the 4000 pg/mL standard working solution into the first EP tube and mix thoroughly to make a 2000 pg/mL standard working solution. Repeat this procedure for subsequent tubes. The last tube is directly used as a blank well, and there is no need to draw liquid from the penultimate tube, as shown in the figure below.
|