- 5-HIAA ELISA Kit

- Product Detail
- Company Profile
Product Specification
| Usage | I. Sample Collection, Preparation, and Storage 1. Serum: After placing whole blood samples at room temperature for 2 hours or at 4°C overnight, centrifuge at 1000×g for 20 minutes. Remove the supernatant for testing. Blood collection tubes should be disposable, pyrogen-free, and endotoxin-free. Store at -20°C or -80°C and avoid repeated freeze-thaw cycles. 2. Plasma: Within 30 minutes of collection, centrifuge at 1000×g for 15 minutes at 2-8°C. Remove the supernatant for testing. EDTA-Na2 is recommended as an anticoagulant. Avoid using samples with hemolysis or high lipid profiles. Store at -20°C or -80°C and avoid repeated freeze-thaw cycles. 3. Tissue Homogenization: Take an appropriate amount of tissue and wash it in pre-chilled PBS (0.01M, pH 7.0-7.2) to remove blood (lysed red blood cells in the homogenate will affect the measurement results). After weighing, mince the tissue and mix it with the appropriate volume of PBS (generally a 1:9 weight-to-volume ratio; the specific volume can be adjusted according to experimental needs and recorded. It is recommended to add protease inhibitors to the PBS). Pour the mixture into a glass homogenizer and grind thoroughly on ice. To further lyse tissue cells, the homogenate can be ultrasonically disrupted or repeatedly freeze-thawed (keep the homogenate in an ice bath during ultrasonication, and repeat the freeze-thaw cycle twice). Finally, centrifuge the homogenate at 5000 × g for 5-10 minutes. The supernatant is then collected for analysis. 4. Cell Culture Supernatant: Centrifuge the cell supernatant at 1000 × g for 20 minutes to remove impurities and cell debris. Remove the supernatant for testing and store at -20°C or -80°C, but avoid repeated freezing and thawing. 5. Urine: Collect the first morning urine (midstream) or 24-hour urine collection. Centrifuge at 2000×g for 15 minutes, collect the supernatant, and store the sample at -20°C. Avoid repeated freezing and thawing. 6. Saliva: Collect the sample using a saliva collection tube, then centrifuge at 1000×g for 15 minutes at 2-8°C. Remove the supernatant for testing, or aliquot and store at -20°C. Avoid repeated freezing and thawing. 7. Other biological samples: Centrifuge at 1000×g for 20 minutes, collect the supernatant, and store the sample at -20°C. Notes: 1. Samples should be clear and transparent, and suspended matter should be removed by centrifugation. Hemolysis of the sample can affect the results, so hemolyzed samples should not be used. 2. Samples can be stored at 4°C if tested within one week of collection. If testing cannot be performed promptly, aliquot the sample into single-use portions and freeze at -20°C (for testing within one month) or -80°C (for testing within three to six months). Avoid repeated freeze-thaw cycles. Bring samples to room temperature before experimenting. 3. If the concentration of the test substance in your sample is higher than the highest concentration of the standard, perform an appropriate dilution based on the actual concentration (a pilot experiment is recommended to determine the dilution factor). II. Pre-Test Preparation 1. Remove the test kit from the refrigerator 30 minutes in advance and equilibrate to room temperature. 2. Dilute 25 μg of concentrated wash buffer to 1 μg of working solution with double-distilled water. Return any unused solution to 4°C. 3. Standards: Add 1.0 mL of Universal Standard & Sample Diluent to the lyophilized standard. Tighten the cap and let stand for 10 minutes to fully dissolve. Then gently mix (concentration 400 ng/mL). Subsequently, serially dilute the standard to 400 ng/mL, 200 ng/mL, 100 ng/mL, 50 ng/mL, 25 ng/mL, 12.5 ng/mL, and 6.25 ng/mL. Use the standard diluent (0 ng/mL) as a blank well. Prepare the required amount of standard and set aside. It is recommended that the prepared standard be added to the sample within 15 minutes; it is not recommended to allow the sample to sit for extended periods. 4. Biotin Conjugate Working Solution (1x): Centrifuge before opening the bottle. Dilute with Biotin Conjugate Diluent immediately before use. Prepare the total volume required for each experiment (50 μL per well) based on the pre-calculated volume. Add 0.1-0.2 mL more, for example, 10 μL of biotin conjugate to 990 μL of biotin conjugate diluent. Mix gently and mix thoroughly. Prepare within one hour of use. 5. Streptomycin-Horseradish Peroxidase Conjugate Working Solution (1x): Centrifuge before opening the bottle. Dilute with enzyme conjugate diluent immediately before use. Prepare the pre-calculated total volume required for each experiment (100 μL per well). Prepare an extra 0.1-0.2 mL. For example, prepare 10 μL of enzyme conjugate to 990 μL of enzyme conjugate diluent. Mix gently. Prepare within one hour of use. 6. TMB Substrate - Pipette the required amount of solution. Do not pour any remaining solution back into the reagent bottle. Note: 1. Ensure all components of the kit are dissolved and mixed thoroughly before use. Discard any remaining standard after reconstitution. 2. Concentrated biotin conjugates and concentrated enzyme conjugates are relatively small and may disperse throughout the tube during transportation. Before use, centrifuge at 1000 × g for 1 minute to allow any liquid on the tube walls or cap to settle to the bottom. Mix the solution by carefully pipetting 4-5 times before use. Prepare the standard, biotin conjugate working solution, and enzyme conjugate working solution according to the required volume and use the corresponding diluents. Do not mix them. 3. Concentrated wash buffer may crystallize after removal from the refrigerator. This is normal. Dissolve the crystals completely in a water bath or incubator before preparing the wash buffer (do not heat above 40°C). The wash buffer should be at room temperature before use. 4. Samples should be added quickly, preferably within 10 minutes for each addition. To ensure accuracy, replicate wells are recommended. When pipetting reagents, maintain a consistent order of addition from well to well. This will ensure consistent incubation times for all wells. 5. During the wash process, any remaining wash solution in the reaction wells should be patted dry on absorbent paper. Do not place filter paper directly into the reaction wells to absorb water. Before reading, be sure to remove any remaining liquid and fingerprints from the bottom of the wells to avoid affecting the microplate reader reading. 6. The color developer, TMB, should be protected from direct sunlight during storage and use. After adding the substrate, carefully observe the color change in the reaction wells. If a gradient is already evident, terminate the reaction early to prevent excessive color from darkening and affecting the microplate reader reading. 7. All test tubes and reagents used in the experiment are disposable. Reuse is strictly prohibited, as this will affect the experimental results. 8. Wear a lab coat and latex gloves for proper protection during the experiment, especially when testing blood or other body fluid samples. Please follow the national biological laboratory safety regulations. 9. Components from different batches of the kit should not be mixed (except for the wash solution and the reaction stop solution). 10. The enzyme labeling strips in the kit are removable. Please use them in batches according to experimental needs. III. Procedure 1. Before beginning the experiment, all reagents should be equilibrated to room temperature. Prepare all reagents in advance. When diluting reagents or samples, mix thoroughly, avoiding foaming as much as possible. If the sample concentration is too high, dilute with sample diluent to bring the sample within the detection range of the kit. 2. Sample Addition: Set up separate wells for standards and wells for the sample to be tested. Add 50 μL of the standard or sample to be tested, being careful not to create bubbles. Add the sample to the bottom of the ELISA plate well, minimizing contact with the well walls. Next, add 50 μL of biotin conjugate (1x) to each well. Gently shake to mix thoroughly. Cover the plate or cover with film and incubate at 37°C for 1 hour. 3. To ensure the validity of the experimental results, use a fresh standard solution for each experiment. 4. After a 1-hour incubation, discard all liquid from the wells, spin dry, and wash the plate three times, adding 200 μL of wash buffer (1 x) to each well, soaking for 1-2 minutes each time, and spin dry. 5. Then, add 100 μL of streptomycin-HRP (1 x) to each well, gently shake to mix, cover the plate or cover film, and incubate at 37°C for 1 hour. 6. Discard all liquid from the wells, spin dry, and wash the plate five times, adding 200 μL of wash buffer (1 x) to each well, soaking for 1-2 minutes each time, and spin dry. 7. Add 90 μL of TMB colorimetric reagent to each well and develop at 37°C in the dark for 15-20 minutes (shorten or extend the time depending on the actual color development, but do not exceed 30 minutes). 8. Add 50 μL of stop solution to each well to terminate the reaction (the blue color will immediately turn yellow). The order of adding the stop solution should be the same as the order of adding the substrate solution. To ensure accurate experimental results, add the stop solution as soon as possible after the substrate reaction time has expired. 9. Measure the optical density (OD) of each well sequentially at 450 nm using a microplate reader. Perform the assay within 5 minutes after adding the stop solution. 10. Samples may need to be diluted. Please refer to the sample handling section. Calculation of Results 1. The OD values of the competition standards and samples can be directly substituted into the calculations. If replicates are used, the average value should be used for calculations. 2. For ease of calculation, although concentration is the independent variable and OD value is the dependent variable, the graphs use the OD value of the standard as the horizontal axis (X-axis) and the concentration of the standard as the vertical axis (Y-axis). For intuitive visualization of the experimental results, the graphs present raw data rather than logarithmic values. The OD values of the standard curves may vary due to differences in experimental conditions (such as operator, pipetting technique, plate washing technique, and temperature). The standard curve provided is for reference only. Experimenters need to establish a standard curve based on their own experiments. The OD value of the sample used can be used to calculate the sample concentration on the standard curve, and then multiplied by the dilution factor to obtain the actual concentration of the sample. It is recommended to use professional curve drawing software, such as Curve Expert.
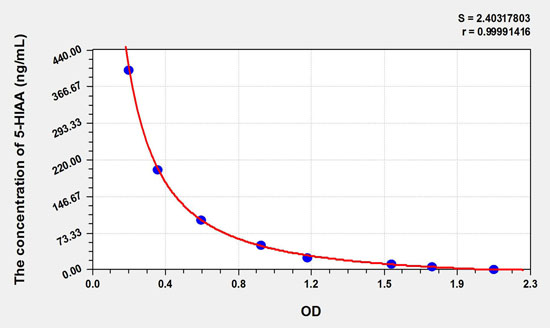 Note: This graph is for reference only. Three samples of known concentration were tested 20 times on each ELISA plate to assess the intra-assay precision. Inter-plate precision (determination of inter-plate precision): CV% <10% Three samples of known concentrations were tested 40 times on three different ELISA plates to evaluate the inter-plate precision. Recovery Recovery experiments were performed by adding 5-HIAA of known concentration to different samples to obtain the range and average recovery.
Linear | 1:2 | 1:4 | 1:8 | 1:16 | ||||||||||||||||||||||||||||||
Serum (n=5) style="text-align: center;" width="102"> 89-97% | 85-94% | 98-105% | 87-98% | 85-92% | 79-96% | ||||||||||||||||||||||||||||||
heparin plasma (n=5) | 89-102% | 88-97% | 88-104% | 97-108% |
Chinese name | 96T | Save conditions |
ELISA plate (removable) | 12 strips × 8 Holes | 4°C/-20°C |
Freeze-dried standards | 2 | 4°C/-20°C |
Standards & Sample Diluent | 20 mL | 4°C/-20°C |
Biotin conjugate (100×) | 60 μL | 4°C/-20°C |
Biotin Conjugate Dilution | 10 mL | 4°C/-20°C |
Concentrated HRP Enzyme Conjugate (100×) | 120 μL | 4°C/-20°C |
Enzyme conjugate diluent | 12 mL | 4°C/-20°C |
Concentrated washing solution (25×) | 20 mL | 4°C/-20°C |
Chromogenic substrate solution (TMB) | 10 mL | 4°C/-20°C (protect from light) |
Reaction stop solution | 6 mL | 4°C/-20°C |
Sealing film | 2 | Normal temperature |
1. If the entire kit is stored at -20°C, please place it at 4°C the night before the experiment.
2. Concentrated wash buffer may precipitate salts if stored at low temperatures. Warm it in a water bath to aid dissolution during dilution.
3. A small amount of water-like substance may appear in the wells of a newly opened ELISA plate. This is normal and will not affect the experimental results.
4. This kit is for laboratory research and development use only and is not intended for use on humans or animals.
5. Reagents should be treated as hazardous substances and should be handled with care and disposed of properly.
6. Always wear gloves, a lab coat, and protective glasses to avoid contact between skin and eyes with the stop solution and TMB. In case of accidental contact, rinse thoroughly with water.
-
AntBio is a biotechnology group company dedicated to serving life sciences, aiming to help scientists accelerate research and improve work efficiency. AntBio provides comprehensive and high-quality reagent tools for basic research, drug development, and diagnosis, including research grade antibodies, proteins, biochemical reagents, and assay kits. These research tools are widely used in different segments of life science research. The group company currently consists of three brands, Absin, Starter-Bio and UA-Bio.
| Request Information |
| Other Products |
| Related Products |
| Recently viewed products |
- SiteMap



