CE Approved FDA(EUA) Covid-19 Antigen Rapid Test .
#ScreeningTest #RapidTest #COVID19 #CoronaVirus #Antigen #AgRapidTest #Covid #Covid19 #CoronaVirus #CoronaTest #CovidTest #CovidTestKit #CovidRecovery #CovidRapid #CovidRapidTest #CovidResponse #RapidTest #RapidTestKit #RapidTestCorona #RapidTestCovid19 #RapidTestKits #Covid19RapidTest #RapidCovid19Test #RapidCovidTesting #DistributorRapidTest
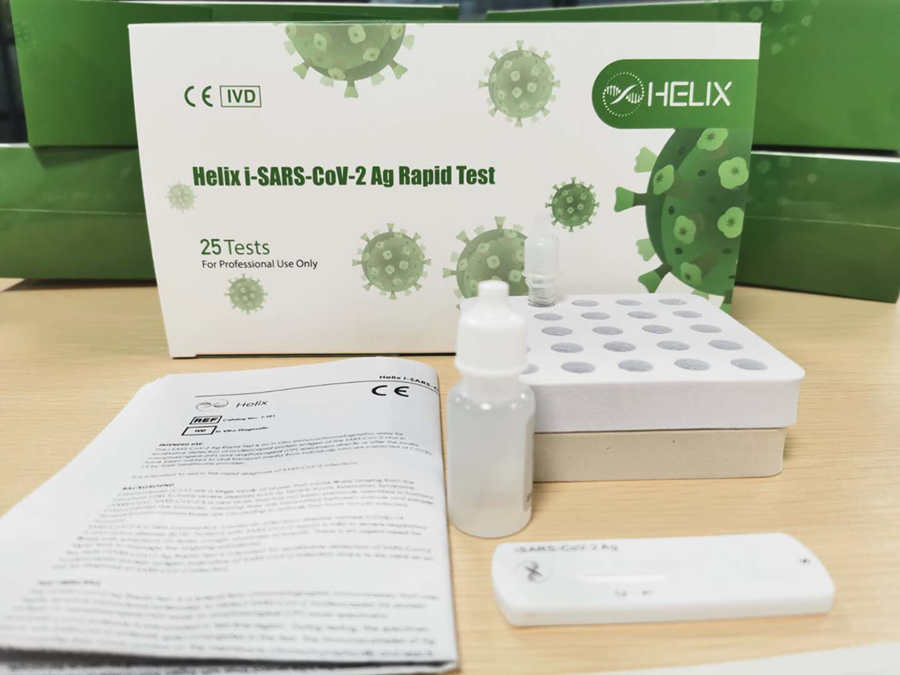
Helix i-SARS-CoV-2 Ag Rapid Test:
* 15 mins to answer, easy to use;
* Adapt to nasopharyngeal swab, oropharyngeal swab;
* Friendly to VTM;
* Designed for mass screen at airport, corporation, hotel and station.
* Correlation with RT-PCR Positive coincidence rate is 88.9%, Negative coincidence rate is 98.3%.
BACKGROUNDINTENDED USE:
The i-SARS-CoV-2 Ag Rapid Test is an in vitro immunochromatographic assay for qualitative detection of nucleocapsid protein antigen of the SARS-CoV-2 virus in nasopharyngeal (NP) and oropharyngeal (OP) specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of COVID-19 by their healthcare provider.
It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections.
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. Entire human populations susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue, dry cough, and loss of taste and smell. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. This test is for detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
TEST PRINCIPLE
The i-SARS-CoV-2 Ag Rapid Test is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid protein from SARS-CoV-2 in nasopharyngeal (NP) swab. The test strip is composed of the following parts: namely sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device. When the sample is added into the sample well, conjugates dried in the reagent pad are dissolved and migrate along with the sample. If SARS-CoV-2 antigen presents in the sample, a complex formed between the anti-SARS-2 conjugate and the virus will be captured by the specific anti-SARS-2 monoclonal antibodies coated on the test line region (T). Absence of the T line suggests a negative result. To serve as a procedural control, a red line will always appear in the control line region (C) indicating that proper volume of sample has been added and membrane wicking has occurred.
TEST PROCEDURE
Allow the test device, test sample and extraction buffer to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove test device from the sealed pouch just prior to the testing and lay flat on work bench.
2. Place the nozzle cap into the sample extraction tube tightly.
3. Reverse the sample extraction tube, and add 2 drops (60-70 μL) of test sample by squeezing the extracted solution tube into the sample window.
4. Wait for the colored band(s) to appear. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
Swab in Viral transport medium(VTM) Test Procedure
1. Mix the sample in VTM by vortexing according the standart lab procedure
2. Collect 300μl swab sample with a calibrated micropipette from the VTM tube, add all collected swab specimen into the sample vial after peel off the sealed pouch.
3. Follow steps 1-4 of the Test Procedure above.
Note: As the sensitivity of this test can be affected by excessive dilution, it is recommended to use 1 ml VTM. Dilution may result in decreased test sensitivity.
Contact
Mail: info@wis-medical.com
WhatsApp/Wechat: +86 16675444525
www.wis-medical.com
bio-equip.cn