INTENDED USE
The Dengue IgG/IgM/NS1 Combo Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of dengue IgG/ IgM and virus antigen (Dengue Ag) in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Dengue viruses. Any reactive specimen with the Dengue IgG/IgM/NS1 Combo Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings.
REAGENTS AND MATERIALS PROVIDED
1.Each foil pouch contains with three items inside:
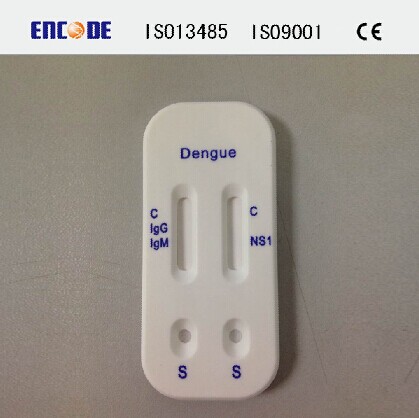
a. One cassette device with Dengue IgG/IgM and Ag NS1 test strips.
b. One plastic dropper.
c. One desiccant.
2.Sample Diluent
3.One package insert (instruction for use).
ASSAY PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: For whole blood samples:
Fill the dropper with the specimen then add 10µL of specimen and 2 drops of buffer into the IgG/ IgM sample well and 2 drops of specimen and 1 drops of buffer into NS1 sample well, making sure that there are no air bubbles.
For Plasma/ Serum samples:
Fill the plastic dropper with the specimen. Holding the dropper vertically, dispense 5 µL of specimen and 2 drops of buffer into the IgG/ IgM sample well and 2 drop of specimen and 1 drops of buffer into the NS1 sample well, making sure that there are no air bubbles.
Step 5:Set up a timer.
Step 6: Results can be read in 15 minutes. Positive results can be visible in as short as 1 minute.
Don’t read results after 30 minutes. To avoid confusion, discard the test device after interpreting the result.
INTERPRETATION OF RESULTS
Dengue IgG/ IgM Rapid Test:
|
POSITIVE RESULT:
|
IgG Positive:The colored line in the control line region (C) appears and a colored line appears in test line region 1 (G). The result is positive for Dengue virus specificIgG and is probably indicative of secondary Dengue infection.
|
|

|
IgM Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 2 (M). The result is positive for Dengue virus specific-IgM antibodies and is indicative of primary Dengue infection.
|
|

|
IgG and IgM Positive:* The colored line in the control line region (C) appears and two colored lines should appear in test line regions 1 and 2 (G and M). The color intensities of the lines do not have to match. The result is positive for IgG & IgM antibodies and is indicative of secondary Dengue infection.
|
|
*NOTE: The intensity of the color in the test line region(s) (G and/or M) will vary depending on the concentration of Dengue antibodies in the specimen. Therefore, any shade of color in the test line region(s) (G and/or M) should be considered positive.
|
|
NEGATIVE RESULT:

|
The colored line in the control line region (C) appears. No line appears in test line regions 1 or 2 (G or M).
|
|
INVALID RESULT:

|
Control band fails to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
|
Dengue NS1 Rapid Test:
|
POSITIVE RESULT:

|
Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (NS1).
|
|
NEGATIVE RESULT:

|
Only one colored band appears in the control region (C). No apparent colored band appears in the test region (NS1).
|
|
INVALID RESULT:

|
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
|
PERFORMANCE CHARACTERISTICS
Clinical Performance
Dengue IgG/IgM Rapid Test:
The specimens obtained from a population of symptomatic and asymptomatic individuals. Results were confirmed by a leading commercial Dengue ELISA test.
Dengue IgG/IgM Rapid Test vs. ELISA
|
Dengue Infection
|
Result
|
Ig M
|
IgG
|
|
Primary Infection
|
Positive
|
14
|
0
|
|
Negative
|
3
|
17
|
|
Total
|
17
|
17
|
|
Relative Sensitivity
|
82.4%
|
0%
|
|
Secondary Infection
|
Positive
|
39
|
55
|
|
Negative
|
16
|
0
|
|
Total
|
55
|
55
|
|
Relative Sensitivity
|
70.9%
|
>99.0%
|
|
Non-Dengue Infection
|
Positive
|
0
|
0
|
|
Negative
|
378
|
378
|
|
Total
|
378
|
378
|
|
Relative Specificity
|
>99.0%
|
>99.0%
|
For the primary and secondary infection, the overall sensitivity is 95.8%, the overall specificity is >99.0% and the overall accuracy is 99.3%.
Dengue NS1 Rapid Test:
A total of 114 patient samples from susceptible subjects were tested by the Dengue NS1 Rapid Test Strip and by a commercial EIA. Comparison for all subjects is showed in the following table:
|
|
Dengue NS1 Rapid Test
|
|
|
Dengue Ag EIA Test
|
Positive
|
Negative
|
Total
|
|
Positive
|
66
|
3
|
69
|
|
Negative
|
2
|
43
|
45
|
|
Total
|
68
|
46
|
114
|
Relative Sensitivity: 95.6%, Relative Specificity: 95.5%, Overall Agreement: 95.6%
bio-equip.cn