|
Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tumor Diagnosis and Treatment
hits:24 Date:02/13/26
1. Concept
Pheochromocytoma and paraganglioma (PPGL) are key subtypes of neuroendocrine tumors (NETs), originating from the adrenal medulla and extra-adrenal sympathetic nerve chains, respectively. Per the latest WHO classification, all PPGLs are considered to have metastatic potential, replacing the traditional benign/malignant dichotomy with "metastatic" and "non-metastatic" categorizations. Chromogranin A (CgA), a prominent member of the chromogranin family, is an acidic soluble protein widely distributed in neuroendocrine tissues. It is co-synthesized, stored, and released with catecholamines in sympathetic nerve terminal granules, making it a pivotal serum biomarker for NETs—particularly valuable for addressing the limitations of traditional diagnostic indicators in evaluating tumor burden and prognosis.
2. Research Frontiers
2.1 Disease Characteristics and Diagnostic Challenges of PPGL
PPGL presents unique clinical and diagnostic complexities:
Metastatic Potential: All PPGLs carry metastatic risk, with recurrence or metastasis potentially occurring years to decades post-initial treatment, necessitating long-term follow-up.
Limitations of Traditional Markers: Urinary catecholamines and other conventional indicators effectively assess functional status but fail to reliably evaluate tumor burden, prognosis, or treatment response—creating an urgent need for more robust biomarkers.
2.2 Biological Characteristics and Detection Methods of Chromogranin A
CgA’s properties and detection systems support its clinical utility:
Tissue Distribution: Ubiquitous in neuroendocrine tissues, CgA is specifically expressed in neuroendocrine cells, providing high tissue specificity for NET identification.
Detection Technology: The primary method is double-antibody sandwich enzyme-linked immunosorbent assay (ELISA), which enables precise quantitative analysis via specific antibody recognition and enzymatic colorimetry. The typical clinical reference range for serum CgA is 27–94 ng/mL.
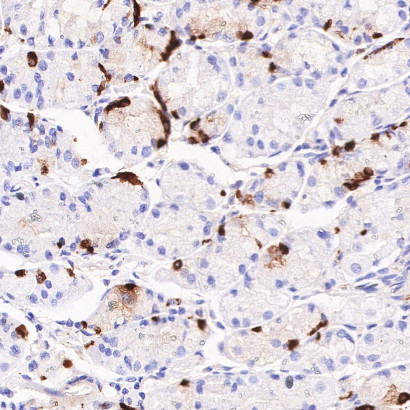
2.3 Association Between CgA and Clinicopathological Features of PPGL
Clinical studies highlight CgA’s value in stratifying PPGL patients:
Metastasis Correlation: Serum CgA levels are significantly elevated in patients with distant metastases, while no significant correlation is observed with patient gender, age, or tumor functional status.
Tumor Type Coverage: CgA levels show no statistical difference between adrenal pheochromocytomas and extra-adrenal paragangliomas, confirming its broad applicability across PPGL subtypes.
Metastatic Risk Assessment: These characteristics make CgA a unique and reliable marker for evaluating PPGL metastatic potential.
2.4 Role of CgA in Treatment Efficacy Evaluation
CgA is a robust tool for monitoring response to PPGL therapies:
131I-MIBG Therapy Monitoring: Per RECIST criteria, patients with effective treatment (complete response, partial response, stable disease) maintain low or undetectable serum CgA levels. In contrast, disease progression is associated with a significant rise in CgA.
Dynamic Tracking: CgA’s close correlation with treatment outcomes enables real-time assessment of therapeutic efficacy and early detection of disease progression, guiding timely treatment adjustments.
2.5 Clinical Significance of CgA in Prognosis Assessment
CgA levels are strongly linked to PPGL patient prognosis:
Tumor Burden Indicator: High CgA expression correlates with increased tumor burden and elevated risk of disease progression.
Postoperative Monitoring: Non-metastatic patients typically show a significant decrease in serum CgA post-surgery, while metastatic patients maintain elevated levels.
Combined Diagnostic Value: When integrated with other clinical indicators, CgA improves diagnostic sensitivity, providing comprehensive insights for clinical decision-making.
2.6 Clinical Application Prospects and Limitations of CgA Testing
While CgA is valuable, its clinical use requires awareness of limitations:
Limitations: CgA levels can be falsely elevated by medications (e.g., proton pump inhibitors) and renal insufficiency, necessitating correlation with patient medication history and renal function.
Interpretation Guidelines: Results must be combined with clinical manifestations, imaging studies, and other laboratory data for comprehensive judgment.
Future Directions: Research should focus on CgA’s role in guiding personalized treatment and predicting early recurrence, expanding its clinical utility.
3. Research Significance
CgA addresses a critical unmet need in PPGL management by serving as a reliable biomarker for metastatic risk assessment, treatment efficacy monitoring, and prognosis evaluation. Its ability to reflect tumor burden and disease progression complements traditional diagnostic methods, enabling more precise patient stratification and personalized care. For NETs beyond PPGL, CgA’s broad expression in neuroendocrine tissues makes it a versatile tool for diagnosis and monitoring. Standardized CgA testing and rational interpretation support long-term follow-up of PPGL patients, ultimately improving clinical outcomes and reducing mortality associated with metastatic disease.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
CgA’s role as a NET biomarker stems from its biological function: As a secretory protein stored in neuroendocrine granules, it is released into the bloodstream alongside hormones (e.g., catecholamines) during tumor cell activation or proliferation. Elevated serum CgA reflects increased neuroendocrine cell activity or tumor burden, providing a direct link to disease status.
4.2 Research Methods
Key methods for studying CgA include:
Quantitative Detection: ELISA for serum CgA quantification, the gold standard for clinical monitoring.
Qualitative and Localization Analysis: Immunohistochemistry (IHC) to detect CgA expression in tissue samples, aiding NET diagnosis and differentiation.
Correlative Studies: Statistical analysis of CgA levels with clinicopathological features (e.g., metastasis, tumor size) and treatment outcomes to validate its clinical utility.
4.3 Product Applications
ANT BIO PTE. LTD.’s Chromogranin A antibodies, led by the STARTER brand’s "S-RMab® Chromogranin A Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2192), are essential tools for NET research and clinical diagnostics:
Neuroendocrine Tumor Diagnosis: Enables qualitative diagnosis of pituitary adenomas, islet cell tumors, pheochromocytomas, carcinoids, and other NETs.
Neuroendocrine Differentiation Identification: Detects neuroendocrine differentiation in non-neuroendocrine tumors (e.g., prostate cancer, lung cancer), assessing tumor heterogeneity and prognosis.
Neuroendocrine System Research: Supports studies on the distribution and function of normal neuroendocrine cells, peptidergic neurons, and the diffuse neuroendocrine system.
Treatment Efficacy Monitoring: Serves as a histological validation tool for serological CgA results, assisting in evaluating treatment response and disease progression.
The S0B2192 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant rabbit monoclonal platform and validated for IHC, offers exceptional advantages: high specificity with clear cytoplasmic granular localization (ensuring reliable neuroendocrine cell identification in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community with high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in oncology, pathology, and neuroendocrinology. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through precise diagnosis and cutting-edge life science research.
6. Related Product List
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
|