|
Ferroptosis Signaling Pathway: Panoramic Analysis of Core Mechanisms and Research Strategies
hits:37 Date:10/11/25
The mysterious world of cell death welcomes a new member—ferroptosis, which, with its unique iron-dependent and lipid peroxidation characteristics, opens up a new battlefield in the fields of cancer therapy and disease intervention.
Ferroptosis is an iron-dependent, lipid peroxidation-driven form of programmed cell death. Since its official naming in 2012, it has become a research hotspot in tumor suppression, neurodegenerative diseases, and metabolic diseases. Different from apoptosis and necrosis, ferroptosis exhibits unique characteristics in cell morphology, biochemistry, and genetics, mainly manifested as mitochondrial shrinkage, increased membrane density, and accumulation of lipid peroxides. This article will systematically analyze the core signaling pathways, key regulatory factors, advanced research strategies, and targeted therapeutic prospects of ferroptosis, providing comprehensive guidance for related research.
I. Core Biochemical Basis of Ferroptosis
The occurrence of ferroptosis depends on three key biochemical processes: iron metabolism imbalance, lipid peroxidation, and failure of the antioxidant defense system. These three processes are intertwined and collectively constitute the core biochemical basis of ferroptosis.
As a catalyst for the Fenton reaction, iron can convert hydrogen peroxide into highly reactive hydroxyl radicals, thereby triggering lipid peroxidation. Under normal circumstances, intracellular iron homeostasis is precisely regulated by transferrin receptor (TfR), ferroportin (FPN), and other proteins. When ferrous iron (Fe²⁺) accumulates excessively in cells, it generates a large amount of reactive oxygen species (ROS) through the Fenton reaction, which attacks polyunsaturated fatty acids (PUFAs) on the cell membrane. PUFAs are synthesized into polyunsaturated fatty acid phospholipids (PUFA-PLs) under the action of acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3), and then oxidized by lipoxygenase to generate phospholipid hydroperoxides (PUFA-PL-OOHs), ultimately leading to cell membrane rupture and cell death.
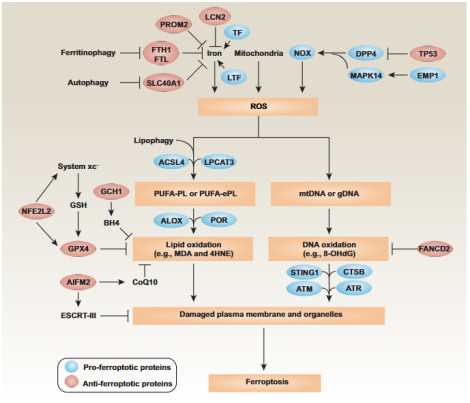
Figure 1 Ferroptosis Signaling Pathway [1]
II. Key Signaling Pathway Network
1. GPX4 Core Regulatory Pathway
Glutathione peroxidase 4 (GPX4) is the most core negative regulator of ferroptosis. GPX4 relies on glutathione (GSH) to reduce lipid peroxides to harmless lipid alcohols, thereby inhibiting the accumulation of lipid peroxidation. The cystine/glutamate antiporter system Xc⁻ (composed of SLC7A11 and SLC3A2 subunits) is responsible for transporting extracellular cystine into the cell while transporting glutamate out of the cell. Intracellular cystine is then reduced to cysteine, which is used for GSH synthesis. When the activity of system Xc⁻ is inhibited (e.g., using Erastin) or the activity of GPX4 is directly inhibited (e.g., using RSL3), the function of GPX4 is lost, triggering ferroptosis.
2. GPX4-Independent Backup Systems
In recent years, studies have found that there are also GPX4-independent regulatory mechanisms for ferroptosis:
Ferroptosis suppressor protein 1 (FSP1): Catalyzes the conversion of ubiquinone (CoQ) to ubiquinol (CoQH₂), which directly neutralizes lipid free radicals and forms an independent antioxidant system on the plasma membrane.
GTP cyclohydrolase 1 (GCH1): Produces tetrahydrobiopterin (BH4), an effective antioxidant that inhibits lipid peroxidation.
Dihydroorotate dehydrogenase (DHODH): Inhibits ferroptosis in mitochondria through a mechanism similar to FSP1.
The following table summarizes the main regulatory factors of ferroptosis and their functions:
Table: Key Regulators of Ferroptosis and Their Functions
| Regulator |
Function |
Regulatory Direction |
| GPX4 |
Reduces lipid peroxides |
Negative Regulation |
| System Xc⁻ (SLC7A11) |
Cystine uptake, GSH synthesis |
Negative Regulation |
| FSP1 |
Ubiquinol regeneration, neutralizes lipid free radicals |
Negative Regulation |
| GCH1 |
Produces BH4, exerts antioxidant effect |
Negative Regulation |
| ACSL4 |
PUFA-PLs synthesis |
Positive Regulation |
| NOXs |
ROS production |
Positive Regulation |
| NANS |
Inhibits NF-κB-FTH1 pathway |
Positive Regulation |
3. Emerging Regulatory Nodes: NANS and NOXs
Latest studies have found that N-acetylneuraminic acid synthase (NANS) plays an important role in colorectal cancer ferroptosis by regulating iron homeostasis. NANS directly binds to the N-terminal domain of TAK1, blocks the formation of the TAK1-TAB1 complex, inhibits K63 ubiquitination and phosphorylation of TAK1, and thereby inhibits the downstream NF-κB signaling pathway. The downregulation of the NF-κB signaling pathway reduces its transcriptional activation of ferritin heavy chain 1 (FTH1). As an iron storage protein, the decreased expression of FTH1 leads to an increase in intracellular free iron, promoting the occurrence of ferroptosis. Members of the NADPH oxidase (NOXs) family (NOX1, NOX2, and NOX4) have also been confirmed to promote ferroptosis through different mechanisms. NOX1 binds to dipeptidyl peptidase 4 (DPP4) under the regulation of p53 protein to mediate plasma membrane lipid peroxidation; NOX4 is induced and expressed by the EGFR-MAPK signaling pathway, producing H₂O₂ to participate in the Fenton reaction.
III. Ferroptosis Research Strategies and Technologies
1. Gene Screening and Molecular Identification
CRISPR-Cas9 genome-wide screening is a powerful tool for discovering new regulatory factors of ferroptosis. For example, researchers identified NANS as a positive regulatory factor of ferroptosis through metabolic enzyme library screening. RNA interference technology can be used for targeted verification of specific gene functions. A study screened through a synthetic siRNA library targeting the HDACs family (HDAC1-11) and found that silencing HDAC1 can significantly promote Erastin-induced ferroptosis. Molecular biology techniques such as chromatin immunoprecipitation (ChIP) can be used to study the binding of transcription factors to target gene promoters. Studies have shown that the NF-κB subunit RelA can directly bind to the FTH1 promoter, and NANS deficiency enhances this binding.
2. Cell Death Confirmation and Phenotypic Analysis
Ferroptosis-specific inhibitors are key tools for confirming ferroptosis. Ferrostatin-1 and Liproxstatin-1 are commonly used ferroptosis inhibitors; if they can reverse cell death, it indicates that the mode of cell death may be ferroptosis.
Lipid peroxidation detection is a core indicator for ferroptosis characterization. The C11-BODIPY⁵⁸¹/⁵⁹¹ probe can be used to detect intracellular lipid peroxidation levels.
Intracellular iron ion detection can use fluorescent probes such as Phen Green SK, RPA, and RhoNox-1.
Electron microscopy observation can reveal the unique ultrastructural characteristics of ferroptosis: mitochondrial shrinkage, increased membrane density, and decreased or absent cristae.
3. Advanced Imaging Technologies
Latest studies have shown that fluorescence lifetime imaging microscopy (FLIM) combined with polarity-sensitive fluorescent probes (e.g., SBD-CH) can real-time monitor changes in endoplasmic reticulum polarity during ferroptosis, providing a new method for early detection. The advantage of this technology is that fluorescence lifetime is an inherent property of fluorescent molecules, independent of probe concentration, which can avoid imaging distortion caused by traditional intensity-based probes.
IV. Disease Association and Prospects of Targeted Therapy
1. A Double-Edged Sword in Cancer Therapy
Ferroptosis plays a dual role in cancer: on the one hand, inducing ferroptosis can inhibit tumor growth; on the other hand, some tumor cells can acquire ferroptosis resistance by upregulating antioxidant pathways. In colorectal cancer, the expression level of NANS is significantly correlated with patient prognosis. Studies have found that under ferroptosis stress, CDK1-mediated phosphorylation of NANS at serine 275 (S275) triggers UBE2N-dependent ubiquitin-mediated degradation, leading to the activation of the NF-κB-FTH1 axis and ferroptosis resistance. The combined use of CDK1 inhibitors (e.g., RO-3306) and ferroptosis inducers has shown a synergistic anti-metastatic effect in animal models.
2. Non-Alcoholic Fatty Liver Disease (NAFLD/NASH)
Ferroptosis plays a key role in the occurrence and development of NAFLD/NASH. Bioinformatics analysis identified 9 NASH ferroptosis key genes including ZFP36, ATF3, SOCS1, IL-6, PTGS2, and JUN. Experiments confirmed that in HepG2 cells treated with free fatty acids (FFA), the expression levels of c-jun, ZFP36, ATF3, and IL-6 proteins increased, and lipid peroxidation was enhanced. Inhibiting ferroptosis can almost completely inhibit the occurrence of NASH.
3. Neurodegenerative Diseases
In neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease, iron accumulation and lipid peroxidation are involved in the process of neuronal death. The inactivation of GPX4 leads to the accumulation of lipid peroxides (e.g., 4-HNE), triggering the destruction of neuronal membrane structure. The synergistic activation of transcription factors AP-2γ and SP1 can upregulate the expression of GPX4, and this pathway provides a new target for neuroprotection.
V. Challenges and Future Directions
Current ferroptosis research faces many challenges: the crosstalk between ferroptosis and other cell death modes is complex; the tissue-specific regulatory mechanisms are still unclear; the delivery efficiency and specificity of targeted drugs are insufficient. Future research focuses should include: developing spatiotemporally specific inducers; exploring tissue-specific regulatory networks; optimizing nanoparticle delivery systems to improve targeting; and in-depth studying the regulatory mechanisms of post-translational modifications (e.g., phosphorylation, ubiquitination, acetylation). With the deepening understanding of ferroptosis mechanisms and the continuous innovation of research technologies, therapeutic strategies targeting ferroptosis are expected to achieve breakthrough progress in the fields of cancer, neurodegenerative diseases, and metabolic diseases.
References
[1] Signaling pathways and defense mechanisms of ferroptosis. DOI: 10.1111/febs.16059
Recommended Products
| Catalog No. |
Name |
Specification |
| abs580233 |
Reactive Oxygen Species (ROS) Detection Kit (Red Fluorescence) |
500T |
|