Home > News > NEST: The Storage and Transportation Problem of the SARS-CoV-2 Nucleic Acid Detection Kit-Solved!
NEST: The Storage and Transportation Problem of the SARS-CoV-2 Nucleic Acid Detection Kit-Solved!
- The "false negatives" of nucleic acid testing results of the SARS-CoV-2 in some samples have pushed the detection kit and RT-PCR technology to a climax of public opinion, but few people have paid attention to the cold chain transportation of the kit.
In order to solve the problem that reagents need to be stored and transported with high requirements, Wuxi Techstar Technology Co., Ltd. launched a new Product- StarFD SARS-CoV-2 RT-PCR Assay.
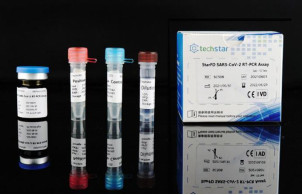
Product Introduction
This kit is a freeze-dried reagent test SARS-CoV-2, used for in vitro qualitative detection of SARS-CoV-2 ORF1ab and N genes in nasopharyngeal swabs, saliva and other samples of patients with suspected cases of virus infection; It is specifically designed for screening in particular groups , each assay contains 96 freeze-dried reagents.
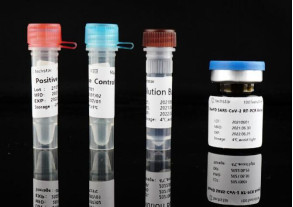
Intended use
This kit is used to qualitatively detect the ORF1ab and N genes of SARS-CoV-2 virus RNA extracted from nasopharyngeal swabs, oropharyngeal swabs and sputum of suspected clustered cases and other suspected infected patients who need to be diagnosed.
The current problems of the SARS-CoV-2 Liquid detection kit
Transportation problem:
Because it contains biologically active ingredients (Taq enzyme, reverse transcriptase, etc.), liquid nucleic acid detection reagents generally need to be stored and transported in an environment of about -20°C, which greatly increases transportation costs, even if cold chain transportation is used, the transportation time generally should not exceed 7 days, too long transportation time will affect the dispensing of liquid reagents.
Storage problem:
In some areas, frequent power shortages and other emergency power failures of power facilities may lead to reagent failure and can not be used anymore.
Insufficient storage space:
Under normal circumstances, medical institutions will not be equipped with too many refrigerators or large areas of cold storage when they only provide routine molecular testing items. During the epidemic, they cannot meet the storage needs of a large number of SARS-CoV-2 testing reagents.
Application of all-component freeze-drying process in nucleic acid detection reagents
Advantages and features:
1. Convenient transportation: The reagents are freeze-dried. Compared with normal liquid PCR reagents, they can be transported at room temperature, which greatly reduces transportation costs;
2. Convenient storage: Freeze-Dried reagents can be stored at room temperature for one year; compared with normal liquid PCR test kits that need to be stored strictly at -20±5°C, storage is more flexible;
3. Easy to use: The reagents can be divided and used after adding the dilution buffer to reconstitute, no reagent preparation and packaging are required, which greatly increases the detection efficiency
4. High sensitivity: up to 200copies/mL;
5. It can be adapted to different types of PCR instruments, such as ABI7500, LightCycler480, etc.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- CD182 Antibodies: Deciphering CXCR2’s Multifaceted Roles in the Tumor Microenvir 1/29/2026
- Lifeasible Launches Enhanced Bulk Segregant Analysis Service to Boost Plant Bree 1/29/2026
- Eyestem Receives CDSCO Approval to Initiate Phase 2 Randomized, Controlled Human 1/28/2026
- CD172a Antibodies: A Cornerstone Tool for Immune Regulation Research 1/27/2026
- CD170 Antibodies: Blocking Myeloid-Derived Suppressor Cell-Mediated Tumor Immune 1/26/2026
- CD163 Antibodies: Targeted Delivery Systems for Promoting Vascular Regeneration 1/25/2026
- CD14 Antibodies: Deciphering Dual Regulatory Mechanisms in Inflammasome Activati 1/24/2026
- CD146 Antibodies: Targeting Lipid Metabolism and Energy Homeostasis to Intervene 1/23/2026
- Be Prepared for 2026 Weather Extremes Says Cold Chain Technologies 1/23/2026


