Asia’s largest bio event and the new show for Personalised Medicine concluded successfully – full of energy and buzz
In Tokyo, from May 14th to 16th, Asia’s largest bio event “BIOtech Japan 2014” and the new show specialised in Personalised Medicine “1st Personalised Medicine & Diagnostics Expo (IVD Japan 2014)” took place with large attendance from the industry. The 2 shows were full of excitement and energy towards new innovation and business.
Overview
BIOtech Japan is Asia’s largest bio event with 13 years of history and has established a firm position as the annual industry gathering in the Japanese bio-pharma industry. The 2014 show was filled with energy and buzz, fueled by new approaches such as the launch of 1st Personalised Medicine & Diagnostics Expo, featuring the Republic of India as “2014 Partner Country” and conferences covering cutting-edge technologies and industry trends. The success was also reflected by the figures – the number of visitors was 11,074, which was larger than the previous show and also a remarkable number for a purely professional, B to B show specialised in the bio industry. In addition, the number of conference attendees showed 50% increase from the 2013 show.
Key figures:
・572 exhibitors from 20 countries
・11,074 visitors
・210 presentations
・7,269 attendees in the Conference (Keynote, Invited & Special Sessions)
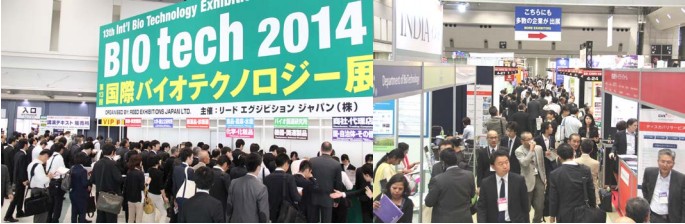
Highlights of the event
■ Stronger commitment by the Japanese and Indian government
This year at BIOtech Japan & IVD Japan 2014, one of the noticeable changes was the stronger commitment by the government. The 2 Keynote Sessions for BIOtech Japan and Personalised Medicine & Diagnostics Expo were the best example of all. The Keynote Speakers included 2 decision makers of Japanese bio-pharma and medical related policies, Mr. Hiroto Izumi, Director-General of the Office of Healthcare Policy, Special Advisor to the Prime Minister, Cabinet Secretariat and Mr. Norihisa Tamura, Minister of Health, Labour and Welfare (spoken by deputy by Mr. Kaname Yamamoto, Director, Office of Medical Devices Evaluation, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare). In addition, BIOtech Japan Keynote Session was also spoken by Dr. K. VijayRaghavan, Secretary to the Government of India, Dept. of Biotechnology, Ministry of Science & Technology.
Featuring the decision makers from Japan and the rapidly-growing bio-pharma market - India, the Keynote Sessions gave a strong impression that 2 the governments are serious about supporting and nurturing the bio-pharma industry.
The Japanese bio industry, like the rest of the world, has suffered difficult conditions in recent years. But now, fueled by the new market development by the discovery of iPS Cells and Regenerative Medicine, new entries from other industries and so on, the market is rejuvenated and the government is again focusing on the industry as a priority sector.
■ Increased number and variety of international exhibitors
Considering the situation a chance, more international companies are setting their target to Japan and exhibiting at BIOtech Japan / IVD Japan. Both the number of overseas exhibitors and the number of exhibiting countries have increased and the show was joined by 2 new national pavilions – India and Korea.
To support those overseas exhibitors, Show Management actively arranged meetings with their target visitors such as business development professionals from pharmaceutical companies and agents/distributors.
Encouraged by the huge success and high satisfaction of existing overseas exhibitors, more pavilions are expected to join next year as well as the existing pavilions coming back with larger space. In addition to the United States decided as “2015 Partner Country”, many other embassies such as Belgium, Czech Republic, Finland, Germany, Singapore, Spain and UK visited the show to see the potential of the show and discuss their participation as a pavilion next year.

■ Huge synergy generated by the launch of new show for Personalised Medicine
1st Personalised Medicine & Diagnostics Expo (IVD Japan 2014) was launched this year to tackle the promising market, personalised medicine. Selected as one of the priority sectors by the Japanese government, a large number of research projects and investments are under way in the field.
As Asia’s only exhibition and conference specialised in diagnostics and testing for personalised medicine, the debut of the show gathered large attention from the industry.
When BIOtech Japan started 13 years ago, the main focus of the event was genomic research. Now the show has added new fields such as regenerative medicine, stem cell research and cell-related products/services in particular. Due to the launch of this new show targeting the rapidly-growing market, more exhibitors with genomic-related products/services are joining again. The synergy between the 2 shows made the entire event more powerful, attracting more lab technicians and genomic-related researchers.
■ 2014 Partner Country: The Republic of India
The first ever “Partner Country” of BIOtech Japan was the Republic of India, which is increasing its presence in the world’s bio-pharma industry. In addition to the India Pavilion organised by ABLE, Dr. K. VijayRaghavan, Secretary to the Government of India, Dept. of Biotechnology, Ministry of Science & Technology spoke in the Keynote Session, addressing “Developing Biotechnology R&D Systems in India -The Challenges for Growing Bioeconomies-“.
India has a long and stable relationship with RIKEN and major Japanese institutions and universities, but the 2 countries have been strengthening this furthermore. Connecting Indian industry leaders and biotech companies with the Japanese bio-pharma industry, the “Partner Country” project served as an accelerator for further cooperation and partnership between the two countries’ bio industries. Dr. VijayRaghavan said “both countries are progressing well in various areas of Biotechnology and can surely benefit from each other’s’ strengths and expertise”, which was proved by the huge attendance and positive feedbacks to the Keynote Session and many active meetings at the India Pavilion.

Conference
This year’s Conference speakers were said to be the best of all times, and sure enough, the number of attendees showed a tremendous growth from the previous show, gathering 7,269 attendees. Not only the number of applications for BIOtech Japan Keynote Session doubled from last year, the total number of attendees to the Conference increased by 50%.
[BIOtech Japan 2014 Keynote Session]
New Growth Strategy -The Future of Medicine Created by Technological Innovation-
<> Our Challenge for Innovation -Building Resilience for Sustainable Growth-
Mr. Yoshihiko Hatanaka, Representative Director, President & CEO, Astellas Pharma Inc.
<> Health Care Policy and New Framework for Promoting Medical Research and Development (in Japan)
Mr. Hiroto Izumi, Director-General of the Office of Healthcare Policy,
Special Advisor to the Prime Minister, Cabinet Secretariat
<> Developing Biotechnology R&D Systems in India -The Challenges for Growing Bioeconomies-
Dr. K. VijayRaghavan, Secretary to the Government of India, Dept. of Biotechnology,
Ministry of Science & Technology, Government of India

[IVD Japan 2014 Keynote Session]
The Key to Realizing Personalised Medicine -Technology Development and Regulations-
<> Japan’s Medical Care Outlook -Projects by the Japanese Ministry of Health, Labour and Welfare-
Mr. Norihisa Tamura, Minister of Health, Labour and Welfare, Ministry of Health, Labour and Welfare
(*spoken by deputy by Mr. Kaname Yamamoto, Director, Office of Medical Devices Evaluation, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare, due to the urgent official duty)
<> Critical Issues Ahead on Our Way to Personalised Medicine
Mr. Chia Feng Lu, Baker & McKenzie (Gaikokuho Joint Enterprise)
<Co-author,"FDA’s regulation of veterinary drug products">
Among 12 lines of Special Sessions, iPS Cells and Regenerative Medicine were the most popular, as well as the new theme, Personalised Medicine.
<Top 5 popular sessions by the number of attendees>
1) BIO-4: Forefront of iPS Cell Research
- Dr. Haruhisa Inoue, Professor, Center for iPS Cell Research and Application (CiRA), Kyoto University
- Dr. Hideyuki Okano, Professor, Dept. of Physiology, Keio University School of Medicine
2) BIO-1: Realizing Regenerative Medicine -From Concept to Clinical Use-
- Dr. Masayo Takahashi, Project Leader, Lab. for Retinal Regeneration Research, RIKEN
- Dr. Katsuhisa Matsuura, Associate Professor, Institute of Advanced Biomedical Engineering and Science (TWIns), Dept. of Cardiology, Tokyo Women’s Medical University
3) BIO-2: The Paradigm Shift in Science Changes Medical Care
- Mr. Yuzo Toda, Director Senior Vice President, FUJIFILM Corp. / Director, FUJIFILM Holdings Corp.
- Mr. Mitsuru Miyata, Executive Leader Writer, Webmaster of Nikkei Biotechnology ONLINE, Nikkei Business Publications, Inc.
4) IVD-1: Developing Strategies for Cancer Care -Outlook for Companion Diagnostics and Personalised Medicine-
- Dr. Wakako Toga, Group Manager, Biomaker&Support Group, Oncology Business Unit, Novartis Pharma K.K.
- Dr. Takayuki Yoshino, Chief, Dept., of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East
5) IVD-2: [Panel Discussion] Issues and Outlook for Personalised Medicine
- Dr. Mayumi Shikano, Director, Pharmaceuticals and Medical Devices Agency
- Dr. Wakako Toga, Group Manager, Biomaker&Support Group, Oncology Business Unit, Novartis Pharma K.K.
- Dr. Takayuki Yoshino, Chief, Dept., of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East
- Mr. Chia Feng Lu, Baker & McKenzie (Gaikokuho Joint Enterprise)
>> Full Program
Academic Forum
What makes BIOtech Japan / IVD Japan special is the Academic Forum, where a large number of academic researchers present their latest research achievements for partnering opportunities. Japanese bio-pharma industry is said to have a huge strength in basic research and eminent platform technologies/seeds, but the same cannot be said about industrializing/commercializing them. That is why the Academic Forum is so important as the partnering platform to connect the academic and industry sector and foster innovation. Attracting a large number of business development professionals from pharmaceutical companies (represented by the Pharma Company Sponsors), biotech companies and other investors, the Academic Forum venue was filled with energy and active discussion.
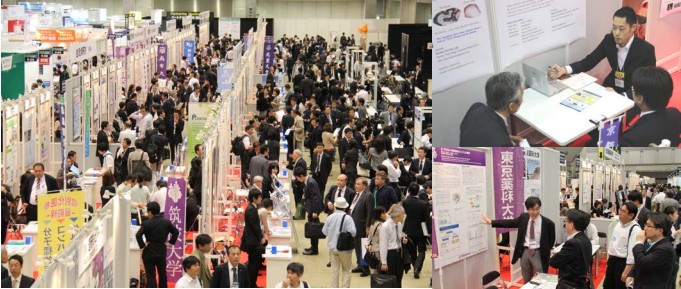
The presentation themes covered various fields, such as Pharmaceutical/Diagnostic Drug Seeds, Drug Discovery Tools, Cancer, Medical/Diagnostic Devices, Personalised Medicine, Regenerative Medicine, Imaging, Immunology, Vaccines, Food, Agriculture and so on.
The followings are the top 5 popular presentations at Academic Forum by the number of attendees.
<Top 5 popular sessions by the number of attendees>
1) Collagen vitrigel: A new biomaterial of highly-dense collagen fibrils useful for tissue regeneration
(Dr. Toshiaki Takezawa, NATIONAL INSTITUTE OF AGROBIOLOGICAL SCIENCES)
2) Cancer stem model cells derived from iPS cells
(Dr. Akifumi Mizutani, OKAYAMA UNIVERSITY)
3) Production and evaluation of crude drug including Glycyrrhiza by hydroponic cultivation system
(Dr. Kayo Yoshimatsu , NATIONAL INSTITUTE OF BIOMEDICAL INNOVATION)
4) The significance of exosome in cancer
(Dr. Ai Kotani, TOKAI UNIVERSITY)
5) Cell-model membranes as a new drug discovery technology
(Dr. Tsutomu Hamada, JAIST)
>> Search from all presentations
Next Show:
New show for Pharmaceutical and Diagnostics R&D – PHARCON Japan
To further enhance the show’s strength in the pharmaceutical-side of life science field, Show Management will launch another show for Pharmaceutical and Diagnostics R&D and renew the whole show as “Life Science World 2015”. Under the new name - Life Science World 2015, there will be 4 events co-held as follows.
Life Science World 2015 consists of:
BIOtech Japan 2015 -- 14th Int’l Bio Technology Exhibition & Conference >>details
PMEX Japan 2015 -- 2nd Int’l Personalised Medicine & Diagnostics Exhibition & Conference >>details
PHARCON Japan 2015 -- 1st Int’l Pharmaceutical and Diagnostics R&D Exhibition & Conference [NEW!]
12th Academic Forum
Dates: May 13-15, 2015
Venue: Tokyo Big Sight, Japan
Organised by: Reed Exhibitions Japan Ltd.
Becoming more comprehensive and more specialised at the same time, the show will attract even more researchers from pharmaceutical companies. The next show aims to gather 650 exhibitors, 250 presentations and 20,000 visitors from all around the world.
For more information, please visit the website: www.bio-t.jp/en/
Contact us:
Life Science World Show Management, Reed Exhibitions Japan Ltd. TEL: +81-3-3349-8519
>>> Press Contact: For coverage / media partnership
Attn: Kyoko Nagakusa (Ms.), International PR
mailto:bio-pr@reedexpo.co.jp
>>> Exhibiting Inquiries
Attn: Masaki Hatabe (Mr.), Chisato Miyawaki (Ms.)
mailto:biotech@reedexpo.co.jp
>>> Visitor Registration (receive the latest information on the next show)
www.bio-t.jp/en/inv/
- International Connect & Expo on Food Science and Technology 3/4/2026
- BIO-Europe Spring® 2026 Charts the Future of Life Sciences from Lisbon’s Sh 2/26/2026
- 2nd International Conference on Cardiology and Cardio Care 2/19/2026
- CPHI & PMEC China 2026: Revolutionizing Laboratory Innovation in Asia 2/13/2026
- 2026 World Radiology and Medical Imaging Conference (2026WRMI) 2/12/2026
- Pharma Partnering EU Summit 2026 2/11/2026
- Pharma Partnering US Summit 2026 2/11/2026
- BioAsia 2026 to Convene the World’s Leading Science, AI, and Industry Pioneers D 2/5/2026
- Global Medical Expo 2026 1/29/2026
- GENAP Summit 2026 1/21/2026



