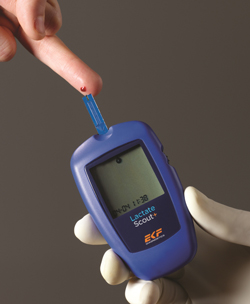
EKF Launches POC Lactate Analyzer with Hematocrit Compensation
Built-in hematocrit compensation delivers high accuracy lactate analysis
|
Cardiff, UK – 20th November 2013 – EKF Diagnostics, the global diagnostics business, announces the launch of the new version of its Lactate Scout+ analyzer. Initially developed to provide coaches and athletes with a precise and easy-to-use mobile lactate test, Lactate Scout’s additional features and functionality – including hematocrit compensation – now mean the device can be used in new medical applications. The in-built compensation for hematocrit (Hct) within Lactate Scout+ ensures accurate lactate measurements even at high (>50%) or low (<35%) Hct levels. The new analyzer’s Hct range is 20-70%, which means patients with either very low Hct levels (e.g. after blood loss) or very high levels (e.g. professional athletes and neonates) can be accurately assessed. This makes Lactate Scout+ ideal for a range of medical applications. These include cardio training, weight reduction and fitness applications which are already well established, as well as new applications in obstetrics. |
|
EKF CEO, Julian Baines commented, “The launch of the new version of Lactate Scout+ reaffirms EKF Diagnostics’ long term expertise in lactate measurement. We are a well-established manufacturer, supplier, distributor and exporter globally of lactate analyzers delivering dependable pre- and post-sale support. We also pride ourselves on developing easy-to-use devices that provide reliable, fast and accurate results.”
About Lactate Scout+
·Results within 10 seconds
·0.2 µL finger prick or earlobe sample
·Hematocrit compensation
·Integrated Bluetooth connectivity for data download
·Stores up to 250 results
For more information on EKF Diagnostics, please visit www.ekfdiagnostics.com.
About EKF Diagnosticswww.ekfdiagnostics.com
Medica 2013, EKF Diagnostics – Hall 3 Stand C70
EKF Diagnostics Holdings plc specializes in the development, production and worldwide distribution of point-of-care blood analyzers for use in the detection and management of diabetes, anemia, lactate and kidney related diseases. Its new Molecular division focuses on molecular and companion diagnostics.
Point-of-care diagnostics: EKF Diagnostics’ expertise covers the entire in vitro diagnostics chain, from fermentation and enzyme production, to liquid reagent manufacture, design and building of world-class diagnostic devices, and distribution of rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Companion Diagnostics: In March 2013 EKF set up a new division to focus on molecular and companion diagnostics - EKF Molecular Diagnostics develops technologies for cancer gene detection. Through its acquisition of UK-based 360 Genomics and by offering innovative products with the potential to change current DNA extraction and detection practices, EKF is addressing the fast growing companion diagnostics market.
EKF Diagnostics products are sold in more than 100 countries around the globe. EKF Diagnostics’ strengths lie in its multi-national research and manufacturing facilities, teams of experienced analysts and engineers in Germany, Ireland, USA and the UK, and a board led by some of world’s foremost authorities in medical diagnostics.
- High Specification Optical Flats for Custom Fizeau Interferometer 12/18/2025
- Merck Launches ChemiSphere® App to Simplify Digital Data Access and Boost L 12/17/2025
- Breathing New Life into Older High-speed Cameras 12/16/2025
- Cold Chain Technologies Expands Industry-Leading Reusable Portfolio 12/11/2025
- Sabin Vaccine Institute’s Investigational Marburg Vaccine Delivered to Ethiopia 12/9/2025
- Cutting-edge Product for Stem Cell Research and Human Embryo Modelling 12/4/2025
- Thermo Fisher Scientific Launches Industry-First, Multi-Parameter Molecular Assa 11/20/2025
- Biotech Fluidics: Solvent Recyclers Improve HPLC System Sustainability 11/20/2025
- Titan Enterprises’ Beverage Flow Meters: Turbine vs. Ultrasonic – Which is Right 11/19/2025
- Gentle and Rapid Detachment of Even Delicate Adherent Cells 11/18/2025