Home > News > Precise Differential Diagnosis of Atypical Cells in Serous Cavity Effusions: The Pivotal Role of CD68 Antibodies
Precise Differential Diagnosis of Atypical Cells in Serous Cavity Effusions: The Pivotal Role of CD68 Antibodies
- 1. Concept
Cytological examination of serous effusions is a routine practice in pathological diagnosis, with core clinical value in detecting malignant tumors, staging known malignancies, and identifying the etiology of effusions of unknown origin. CD68, a specific marker for histiocytes (macrophages), is a key tool for addressing diagnostic uncertainties in these effusions. As a cell surface glycoprotein predominantly expressed in the cytoplasmic granules of histiocytes, CD68 exhibits high specificity for this cell lineage, enabling clear distinction between reactive histiocytes and malignant cells—particularly in cases where morphological features overlap and lead to diagnostic ambiguity.
2. Research Frontiers
2.1 Key Challenges in Cytological Diagnosis of Serous Cavity Effusions
Serous effusion cytology faces significant diagnostic hurdles, primarily in differentiating reactive mesothelial cells, histiocytes, and malignant cells:
Morphological Overlap: Histiocytes may display atypical features such as nuclear enlargement, irregular nuclear contours, nuclear grooves, cytoplasmic vacuolization, and prominent nucleoli—traits that mimic malignant cells.
Structural Confusion: Clustered histiocytes in smears or pseudoglandular structures in cell blocks can be easily misdiagnosed as metastatic carcinoma.
Diagnostic Uncertainty: These ambiguities often result in "atypical" diagnoses, leading to inconsistent clinical treatment strategies and unnecessary patient anxiety or interventions.
2.2 Specificity of CD68 in Histiocyte Identification
CD68’s unique expression pattern provides objective evidence for histiocyte identification:
Expression Profiling: In cases initially diagnosed as "atypical," approximately 71% of cells express CD68, compared to a 62% positivity rate in benign/reactive lesions. This differential expression helps stratify ambiguous cases.
Definitive Classification: Cell populations positive for CD68 but negative for mesothelial (e.g., calretinin) and epithelial (e.g., CEA) markers can be definitively identified as histiocytes. This allows reclassification of approximately 49% of "atypical" cases as benign, resolving diagnostic uncertainty.
2.3 Establishing a Systematic Immunohistochemical Differential Diagnostic Strategy
A standardized workflow leveraging CD68 and a panel of complementary markers optimizes diagnostic accuracy:
Recommended Five-Marker Panel: Comprises two mesothelial markers (calretinin, CK5/6), two epithelial markers (CEA, B72.3), and one histiocyte marker (CD68).
Diagnostic Algorithms:
* Positive mesothelial markers + negative epithelial markers: Reactive mesothelial cells (regardless of CD68 expression).
* Positive epithelial markers + negative CD68: High likelihood of malignancy, requiring additional tissue-specific markers to identify the primary tumor site.
* Positive CD68 + negative mesothelial/epithelial markers: Benign histiocytes.
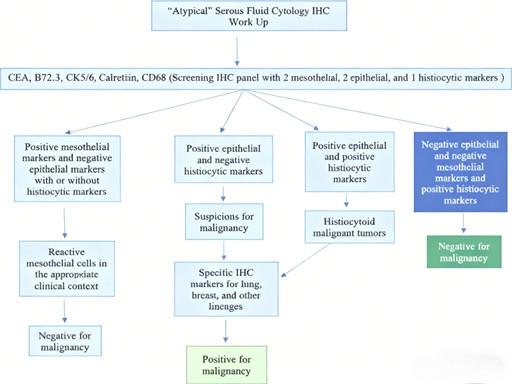
2.4 Unique Value of CD68 in Differentiating Special Tumor Types
CD68 is critical for distinguishing "histiocytoid" malignancies—tumors that mimic histiocyte morphology:
Target Tumor Types: Breast cancer, gastric cancer, and renal cell carcinoma are among the malignancies that may exhibit histiocytoid features.
Dual-Expression Scenario: Rare cases show co-expression of epithelial markers and CD68, indicating histiocytoid malignancy. Further differentiation relies on morphological analysis and tissue-specific markers to avoid misclassifying poorly differentiated carcinomas as benign histiocytes.
2.5 Impact of CD68 Detection on Clinical Decision-Making
CD68 integration into diagnostic workflows significantly improves clinical outcomes:
Enhanced Diagnostic Accuracy: Combined with other markers, CD68 enables reclassification of nearly half of "atypical" cases as benign, eliminating unnecessary follow-up examinations, treatments, and patient burden.
Timely Malignancy Detection: CD68-negative + epithelial marker-positive cases are identified as malignant earlier, facilitating prompt clinical intervention and improving prognosis.
Standardized Treatment Pathways: Objective immunohistochemical evidence reduces inter-observer variability, ensuring consistent treatment strategies across healthcare providers.
2.6 Future Directions of Serous Effusion Diagnostic Technology
Advancements in molecular pathology will drive more precise and personalized serous effusion diagnosis:
Novel Marker Development: Identification of more specific histiocyte markers to further enhance diagnostic sensitivity and specificity.
Quantitative Detection: Adoption of quantitative methods (e.g., digital pathology, flow cytometry) to quantify CD68 expression, reducing subjective interpretation bias.
Multimodal Diagnostic Systems: Integration of morphology, immunohistochemistry, and molecular testing (e.g., next-generation sequencing) to address complex cases and improve diagnostic confidence.
3. Research Significance
CD68 antibodies address a critical unmet need in serous effusion cytology by resolving diagnostic ambiguities associated with atypical cells. By providing a specific marker for histiocytes, CD68 enables accurate differentiation between benign reactive cells and malignant tumors, significantly reducing misdiagnosis rates. This not only optimizes pathological workflows and reduces healthcare costs but also provides reliable evidence for clinical decision-making—ensuring appropriate treatment for malignant cases and avoiding unnecessary interventions for benign ones. Additionally, CD68’s role in identifying tumor-associated macrophages (TAMs) and histiocytic tumors extends its value beyond serous effusion diagnosis to broader oncology and immunology research.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
CD68’s diagnostic utility stems from its tissue-specific expression: It is predominantly localized in the cytoplasmic granules of histiocytes/macrophages, with minimal expression in mesothelial cells, epithelial cells, and malignant tumor cells. This high specificity allows for precise identification of histiocyte populations through antigen-antibody binding, forming the basis for differential diagnosis.
4.2 Research Methods
Key methods leveraging CD68 for serous effusion diagnosis include:
Immunohistochemistry (IHC): The primary technique for detecting CD68 in formalin-fixed paraffin-embedded (FFPE) cell blocks or cytological smears, enabling visualization of expression localization and intensity.
Marker Panel Combination: Concurrent staining with mesothelial, epithelial, and CD68 markers to apply the systematic differential diagnostic algorithm.
Morphological-Immunohistochemical Correlation: Integrating cytological morphology with IHC results to resolve ambiguous cases.
4.3 Product Applications
ANT BIO PTE. LTD.’s CD68 antibodies, represented by the STARTER brand’s "S-RMab® CD68 Recombinant Mouse Monoclonal Antibody" (Catalog No.: S0B2195), are indispensable tools for pathology and research:
Macrophage Identification: Enables precise identification and distribution analysis of macrophages in normal and pathological tissues.
Inflammation and Granulomatous Disease Assessment: Supports evaluation of macrophage infiltration in conditions such as tuberculosis, sarcoidosis, and other inflammatory or granulomatous diseases.
Tumor-Associated Macrophage (TAM) Research: Facilitates detection of TAMs in the tumor microenvironment, analyzing their density, distribution, and correlation with tumor prognosis and treatment response.
Histiocytic Tumor Diagnosis: Serves as an auxiliary diagnostic tool for histiocytic sarcoma, Langerhans cell histiocytosis, and other histiocyte-derived tumors.
Serous Cavity Effusion Diagnosis: Critical for differentiating atypical histiocytes from malignant cells in serous effusions, as part of the recommended five-marker panel.
The S0B2195 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant mouse monoclonal platform and validated for IHC, offers exceptional advantages: high specificity with clear cytoplasmic localization (ensuring reliable macrophage identification in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community with high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in pathology, oncology, and immunology. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through precise diagnosis and cutting-edge life science research.
6. Related Product List
| Catalog No. | Product Name | Host |
| S0B2195 | S-RMab® CD68 Recombinant Mouse mAb (SDT-R146) | Mouse |
| S0B5050 | Alexa Fluor® 647 Mouse Anti-Human CD68 Antibody (S-R657) | Mouse |
| S0B5071 | Mouse Anti-Human CD68 Antibody (S-R657) | Mouse |
| S0B5235 | Rat Anti-Mouse CD68 Antibody (S-R607) | Rat |
| S0B1501 | CD68 Recombinant Rabbit mAb (Alexa Fluor® 594 Conjugate) (S-R025) | Rabbit |
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026
- Precise Differential Diagnosis of Atypical Cells in Serous Cavity Effusions: The 2/9/2026
- Eppendorf Collaborates with Dubai Police to Automate Forensics Laboratories 2/9/2026


