Home > News > Pioneering a New Paradigm in Blood Cancer Treatment: Targeting CD45, the Leukocyte Common Antigen
Pioneering a New Paradigm in Blood Cancer Treatment: Targeting CD45, the Leukocyte Common Antigen
- 1. Concept
CD45, also known as protein tyrosine phosphatase receptor type C (PTPRC) or leukocyte common antigen (LCA), is a transmembrane glycoprotein with a molecular weight of 180–220 kDa. It is universally expressed on all hematopoietic cells except mature erythrocytes, serving as a defining marker for this cell lineage. Structurally, CD45 comprises a highly conserved intracellular phosphatase domain, a transmembrane region, and an extracellular domain. Encoded by the gene located on human chromosome 1q31-32 (containing 35 exons), CD45 generates multiple isoforms through alternative splicing of exons 4/5/6/7. These isoforms exhibit cell-type-specific expression patterns, providing a foundation for immune cell typing. Functionally, CD45 is a key regulator of T cell receptor (TCR) and B cell receptor (BCR) signal transduction, modulating LCK and SRC kinase activity. It also participates in cytokine pathway regulation and maintains lymphocyte survival—making it a central node in hematopoietic cell biology.
2. Research Frontiers
2.1 Biological Characteristics and Functional Mechanisms of CD45
CD45’s functional versatility stems from its structural and genetic properties:
Isoform Diversity: Alternative splicing produces isoforms (e.g., CD45RO, CD45RA, CD45RB) that are differentially expressed on immune cell subsets (e.g., naive vs. memory T cells, B cells, myeloid cells). This specificity enables precise immune cell phenotyping.
Signal Transduction Regulation: As a protein tyrosine phosphatase, CD45 dephosphorylates inhibitory tyrosine residues on SRC family kinases (SFKs), activating TCR/BCR signaling and promoting T/B cell activation. It also modulates cytokine receptor signaling via JAK-STAT pathways, regulating cell proliferation, differentiation, and cytokine production.
Survival and Homeostasis: CD45 contributes to lymphocyte survival and hematopoietic system homeostasis by integrating signals from multiple pathways, ensuring balanced immune responses.
2.2 Key Technical Challenges in CD45-Targeted Therapy
The primary barrier to CD45-targeted therapy is its broad expression across all hematopoietic cells—including therapeutic immune effector cells (e.g., CAR-T cells, hematopoietic stem cells):
"Fratricide" Risk: Traditional targeting strategies would cause therapeutic cells to recognize and eliminate each other, severely compromising treatment efficacy.
Specificity Dilemma: Achieving tumor-specific targeting while sparing healthy hematopoietic cells is challenging due to CD45’s universal expression in the hematopoietic lineage.
Recent breakthroughs using CRISPR-mediated epitope editing have addressed this issue: precise modification of CD45 epitopes on therapeutic cells allows them to retain normal biological functions while evading recognition by CD45-targeted therapies—overcoming the fratricide barrier.
2.3 Current Status and Strategies of CD45-Targeted Drug Development
CD45-targeted drug development focuses on three core directions, with radioimmunoconjugates leading in clinical advancement:
Radioimmunoconjugates (RICs): These agents conjugate CD45 antibodies with radioactive isotopes (e.g., iodine-131, lutetium-177) to precisely target and eliminate CD45-positive tumor cells. Several RICs have entered Phase III clinical trials for acute myeloid leukemia (AML).
Antibody-Drug Conjugates (ADCs): ADCs link CD45 antibodies to cytotoxic payloads, delivering potent anti-tumor agents directly to CD45-positive cells while minimizing off-target toxicity.
Cell Therapy Products: Genetically engineered cell therapies (e.g., CD45-targeted CAR-T cells) combined with epitope-edited donor cells enable safe adoptive cell transfer.
Clinical data for RICs in AML show remarkable efficacy: treated patients maintained complete remission for over 6 months, with 100% successfully undergoing bone marrow transplantation (BMT), compared to only 17% of the control group.
2.4 Breakthrough Applications of Epitope Editing Technology
Epitope editing technology has revolutionized CD45-targeted therapy:
Precision Modification: CRISPR-Cas9-mediated editing alters the amino acid sequence of specific CD45 epitopes, rendering them unrecognizable by therapeutic antibodies while preserving CD45’s natural phosphatase activity and signaling functions.
Therapeutic Cell Protection: Edited T cells and hematopoietic stem cells avoid fratricide by CD45-targeted therapies, maintaining their effector functions (e.g., cytotoxicity, engraftment capacity).
Universal Therapy Potential: This technology enables the development of "off-the-shelf" universal blood cancer treatments, eliminating the need for patient-specific cell manufacturing and expanding access to therapy.
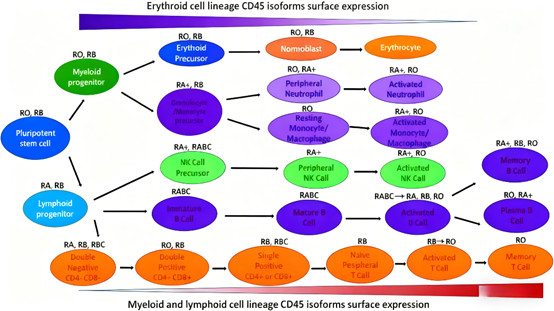
2.5 Significant Clinical Advantages of CD45-Targeted Therapy
CD45-targeted therapy offers multiple clinical benefits in hematological malignancies:
Enhanced Safety: Compared to traditional chemotherapy or radiotherapy, it significantly reduces the incidence of severe complications such as sepsis, infection, and organ toxicity.
Improved Efficacy: Precise targeting of CD45-positive tumor cells enhances clearance efficiency, while reducing damage to healthy tissues.
Facilitated Bone Marrow Transplantation: By reducing tumor burden and conditioning the bone marrow, CD45-targeted therapy enables more patients (previously ineligible due to high tumor load or poor health) to undergo BMT—significantly improving long-term survival.
Broad Applicability: It shows potential across various hematological malignancies, including leukemia, lymphoma, and myeloma, due to CD45’s universal expression in hematopoietic tumors.
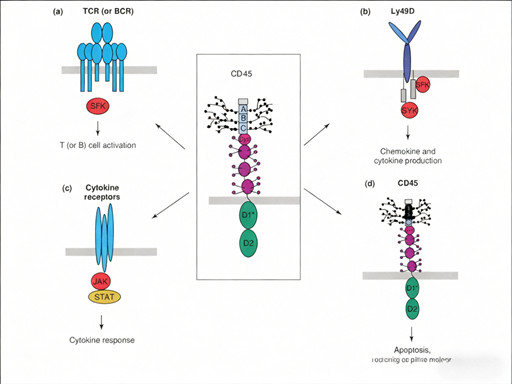
2.6 Future Development Directions for CD45-Targeted Therapy
With advancements in gene editing and antibody engineering, CD45-targeted therapy is evolving toward greater precision, safety, and accessibility:
Optimization of Epitope Editing: Improving editing efficiency, reducing off-target effects, and simplifying manufacturing processes to enhance scalability.
Novel Conjugation Strategies: Developing next-generation RICs/ADCs with improved pharmacokinetics, higher payload potency, and reduced immunogenicity.
Combination Therapies: Exploring synergies with immune checkpoint inhibitors, CAR-T cells, or targeted therapies to amplify anti-tumor effects and overcome resistance.
Personalized Medicine: Tailoring treatment strategies based on disease subtype, CD45 isoform expression, and patient-specific immune profiles to maximize efficacy.
Expanded Indications: Investigating CD45-targeted therapy for non-hematological malignancies with CD45-positive tumor-infiltrating lymphocytes or hematological metastases.
3. Research Significance
CD45-targeted therapy represents a paradigm shift in blood cancer treatment, addressing longstanding challenges in hematological malignancy management. By overcoming the fratricide barrier through epitope editing, it unlocks the therapeutic potential of a universally expressed hematopoietic marker. This approach not only improves treatment efficacy and safety but also expands access to life-saving BMT for previously ineligible patients. Additionally, CD45’s role as a leukocyte marker makes it invaluable for diagnostic applications (e.g., tumor typing, minimal residual disease detection), bridging basic research, clinical diagnosis, and therapy. Insights into CD45’s signaling mechanisms also advance our understanding of hematopoietic biology and immune regulation, benefiting the development of therapies for other immune-related diseases.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
CD45-targeted therapies act through two core mechanisms:
Direct Tumor Cell Killing: RICs/ADCs deliver cytotoxic agents (radiation, toxins) directly to CD45-positive tumor cells, inducing apoptosis or necrosis.
Immune Modulation: By eliminating CD45-positive immunosuppressive cells (e.g., regulatory T cells) and activating anti-tumor immune responses, CD45-targeted therapies reshape the immune microenvironment.
Epitope editing enables safe delivery of these therapies by protecting therapeutic cells from self-targeting, ensuring sustained anti-tumor activity.
4.2 Research Methods
Key research methods for studying CD45 include:
Expression and Phenotyping: Flow cytometry, immunohistochemistry (IHC), and Western blotting to detect CD45 expression and isoform profiles in cells/tissues.
Functional Assays: TCR/BCR signaling assays, kinase activity assays, and cytokine production assays to evaluate CD45’s regulatory role.
Gene Editing and Cell Engineering: CRISPR-Cas9-mediated epitope editing, lentiviral transduction for CAR-T cell generation, and ex vivo expansion of hematopoietic stem cells.
Preclinical and Clinical Evaluation: Xenograft models, patient-derived organoids, and clinical trials to assess therapy efficacy, safety, and pharmacokinetics.
Minimal Residual Disease (MRD) Detection: Flow cytometry or PCR-based assays using CD45 as a marker to monitor residual tumor cells post-treatment.
4.3 Product Applications
ANT BIO PTE. LTD.’s CD45 antibodies, highlighted by the STARTER brand’s "S-RMab® CD45 Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2087), are critical tools for hematology research and clinical diagnostics:
Hematopoietic System Tumor Diagnosis: Enables diagnosis and differential diagnosis of leukemia, lymphoma, and other hematopoietic tumors, distinguishing them from non-hematopoietic malignancies.
Inflammatory Cell Identification: Facilitates qualitative and quantitative analysis of inflammatory cell infiltration in autoimmune diseases, infections, and inflammatory disorders.
Minimal Residual Disease Detection: Serves as a broad-spectrum hematopoietic marker for MRD monitoring post-treatment, enabling early detection of relapse.
Tumor-Infiltrating Lymphocyte (TIL) Assessment: Identifies and quantifies TILs in solid tumor microenvironments, supporting immunotherapy research and patient stratification.
The S0B2087 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant rabbit monoclonal platform and validated for IHC, offers exceptional advantages: high specificity with clear membrane localization (ensuring reliable hematopoietic cell identification in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community with high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in hematology, oncology, and immunology. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through cutting-edge life science research and clinical translation.
6. Related Product List
| Catalog No. | Product Name | Host |
| S0B2087 | S-RMab® CD45 Recombinant Rabbit mAb (SDT-R035) | Rabbit |
| S0B1583 | APC-Cy7 Mouse Anti-Human CD45 Antibody (S-839-3) | Mouse |
| S0B8198 | APC-Cy7 Mouse Anti-Human CD45 Antibody (S-3269) | Mouse |
| S0B8252 | Alexa Fluor® 647 Mouse Anti-Rat CD45 Antibody (S-R591) | Mouse |
| S0B8270 | Biotin Mouse Anti-Rat CD45 Antibody (S-R591) | Mouse |
| S0B0204 | CD45 Recombinant Rabbit mAb (Alexa Fluor® 555 Conjugate) (S-R035) | Rabbit |
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026
- Precise Differential Diagnosis of Atypical Cells in Serous Cavity Effusions: The 2/9/2026
- Eppendorf Collaborates with Dubai Police to Automate Forensics Laboratories 2/9/2026


