Home > News > How to Achieve Precise Selection of IHC Antibodies in Cancer Biomarker Detection?
How to Achieve Precise Selection of IHC Antibodies in Cancer Biomarker Detection?
- Recent Advances
I. Why is Precise Detection of Cancer Biomarkers Necessary?
Biomarkers, as endogenous molecules reflecting normal physiological processes or pathological states, hold significant value in cancer diagnosis, prognosis assessment, and treatment monitoring. By comparing the expression differences of biomarkers in different samples, researchers can deeply analyze the complex mechanisms of disease development. Among various detection technologies, immunohistochemistry has become the preferred method for studying the distribution and localization of biomarkers in the tumor microenvironment due to its unique advantage of spatial resolution.
Unlike traditional detection methods requiring tissue lysis, IHC enables in situ detection of specific biomarkers while preserving tissue structure and cellular morphology. This characteristic allows it to reveal the spatial correlation between biomarker expression levels and histopathological features, providing a unique perspective for understanding cancer biology. However, the accuracy of IHC detection heavily relies on the specificity and reliability of the antibodies used, making the scientific selection and rigorous validation of antibodies particularly important.
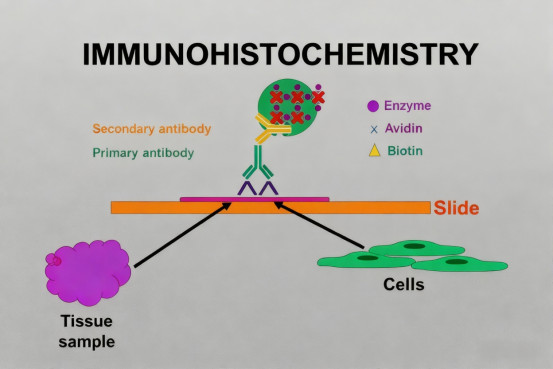
II. What Key Principles Should IHC Antibody Validation Follow?
To ensure the accuracy and reproducibility of IHC results, antibody reagents must undergo systematic validation. Scientific antibody validation should include multiple complementary strategies to comprehensively evaluate the antibody's specificity, sensitivity, and functionality in a specific detection method. Using gene knockout or knockdown cell lines to verify antibody specificity is the primary step, confirming the antibody's ability to bind the target antigen.
Furthermore, it is necessary to confirm the exact epitope recognized by the antibody through mass spectrometry analysis, verify the consistency of detection results using orthogonal methods, and evaluate the antibody's applicability across different sample types. Simultaneously, monitoring inter-batch consistency is crucial, as it directly affects the reliability and reproducibility of experimental results. Establishing standardized validation procedures not only ensures antibody quality but also provides a basis for comparing results between different laboratories.
III. What are the Main Types of Cancer Biomarkers?
Based on their mechanism of action in cancer development, commonly used IHC-detected biomarkers can be divided into several functional categories. Therapeutic target biomarkers, such as Human Epidermal Growth Factor Receptor 2 and Programmed Death-Ligand 1, directly guide clinical treatment decisions. The detection results of these biomarkers are often closely related to the efficacy of specific targeted drugs.
Tumor microenvironment markers include various immune cell markers and stromal component markers, which can reflect the tumor immune status and tissue architecture characteristics. Epithelial-mesenchymal transition-related markers are highly valuable for assessing tumor progression and metastatic potential, involving expression changes in various proteins such as cell adhesion molecules and cytoskeletal proteins. Cell proliferation and apoptosis markers directly reflect the growth characteristics of tumors and are important indicators for assessing tumor malignancy.
IV. How are IHC Antibodies Applied in Tumor Microenvironment Research?
Tumor microenvironment research often requires the simultaneous detection of multiple biomarkers to comprehensively analyze immune cell composition and functional status. By combining IHC antibodies for immune cell markers such as T cells, B cells, and macrophages, the type and distribution of tumor-infiltrating immune cells can be precisely quantified. Combined with spatial analysis techniques, the interaction relationships between immune cells and tumor cells can be further studied.
Beyond cell type markers, the detection of functional status markers is increasingly important. For example, detecting the expression levels of immune checkpoint molecules helps assess the immunosuppressive status, while cell proliferation and activation markers can reflect the functional activity of immune cells. Integrating this information provides an important basis for understanding tumor immune escape mechanisms and predicting immunotherapy responses.
V. What are the Development Trends of IHC Antibody Technology?
With the advancement of precision medicine, IHC antibody technology is developing towards higher specificity and sensitivity. Progress in multiplex detection technology enables the simultaneous detection of multiple biomarkers in a single sample, greatly improving sample utilization and detection efficiency. The rise of computational pathology also provides new tools for analyzing IHC results, allowing for more objective and precise quantification of biomarker expression through artificial intelligence algorithms.
The application of automation technology is transforming traditional IHC detection workflows, improving experimental reproducibility and throughput. The promotion of standardized operations and the refinement of quality control systems help reduce inter-laboratory variations, facilitating the exchange and comparison of research results. These technological advancements collectively propel the application of IHC in cancer research and clinical diagnosis to new heights.
VI. Which Manufacturers Provide IHC Antibodies?
Hangzhou Starter Bio-tech Co., Ltd. (ANT BIO PTE. LTD. group member) 's self-developed "S-RMab® CD21 Recombinant Rabbit Monoclonal Antibody" is a pathology-grade IHC detection antibody characterized by high specificity, excellent sensitivity, and exceptional staining consistency. This product is ideal for applications in B-cell lymphoma diagnosis, follicular dendritic cell network analysis, and immune microenvironment research.
Product Core Advantages:
* High Specificity & Clear Membrane Localization: Precisely recognizes the CD21 (Complement Receptor 2) antigen, demonstrating excellent cell membrane-specific staining in FFPE samples, clearly labeling B cells and follicular dendritic cells (FDCs), with clean background and accurate localization, providing a reliable basis for precise interpretation.
* Excellent Staining Stability & Batch Consistency: Under strict quality control standards, the product exhibits excellent staining stability and minimal batch-to-batch variation, providing clear and reproducible staining results, ensuring stable support for clinical pathological diagnosis and large-scale research projects.
* Suitable Key Application Scenarios: This product is an ideal tool for conducting the following research:
B-Cell Lymphoma Diagnosis & Classification: For differentiating B-cell lymphomas such as Follicular Lymphoma and Chronic Lymphocytic Leukemia, and assisting in assessing the integrity of the follicular dendritic cell network.
Immune Microenvironment & Germinal Center Research: For analyzing the structure and function of germinal centers in lymphoid tissues and studying the role of FDCs in immune responses.
Autoimmune Disease Research: For studying the formation and functional status of lymphoid follicles in diseases like Rheumatoid Arthritis and Systemic Lupus Erythematosus.
Infection & Immunodeficiency Research: For assessing structural abnormalities in lymphoid tissues and immune function status in immunodeficient patients.
* Professional Technical Support: We provide detailed product technical documentation, including complete IHC-P experimental protocols, optimized antigen retrieval methods, and professional interpretation criteria, fully committed to assisting customers in obtaining accurate and reliable results in lymphoma pathological diagnosis and immunology research.
Hangzhou Starter Bio-tech Co., Ltd. (ANT BIO PTE. LTD. group member) is always dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "S-RMab® CD21 Recombinant Rabbit Monoclonal Antibody" or to request a sample test, please feel free to contact us.
Product Information
| Catalog No. | Product Name | Product Parameters |
| S0B2049 | S-RMab® p63 Recombinant Rabbit mAb (SDT-054-38) | Host : Rabbit Conjugation : Unconjugated |
| S0B2309 | S-RMab® ErbB3 Recombinant Rabbit mAb (SDT-991-10) | Host : Rabbit Conjugation : Unconjugated |
| S0B2291 | S-RMab® Cadherin 17 Recombinant Rabbit mAb (SDT-736-23) | Host : Rabbit Conjugation : Unconjugated |
| S0B2043 | S-RMab® CD21 Recombinant Rabbit mAb(SDT-007-47) | Host : Rabbit Conjugation : Unconjugated |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


