Home > News > Plasma p-tau231 Antibodies: Can They Become a New Tool for Early Pathological Detection of Alzheimer's Disease?
Plasma p-tau231 Antibodies: Can They Become a New Tool for Early Pathological Detection of Alzheimer's Disease?
- I. Research Background and Significance: Why are new AD biomarkers needed?
The pathological process of Alzheimer's Disease begins years or even decades before clinical symptoms appear. Therefore, developing biomarkers that can early and accurately identify AD pathology is crucial. Phosphorylated tau protein is one of the core biomarkers for AD, and the tau variant phosphorylated at threonine 231 has shown diagnostic value in cerebrospinal fluid. However, CSF collection is invasive, limiting its widespread application. In recent years, blood-based biomarker detection has become a research hotspot due to its non-invasive and convenient nature. This study aims to develop and validate a novel, ultra-sensitive detection method—Single Molecule Array technology—for the quantitative measurement of plasma p-tau231 and to evaluate its potential for clinical application in the early diagnosis and pathological staging of AD.
II. Research Methods and Cohorts: How was the analytical and clinical performance of plasma p-tau231 validated?
This study employed a multi-phase cohort design, covering the entire disease spectrum from cognitively unimpaired to AD dementia, combined with neuropathological validation, to systematically evaluate the analytical characteristics and diagnostic efficacy of plasma p-tau231.
The research cohorts included:
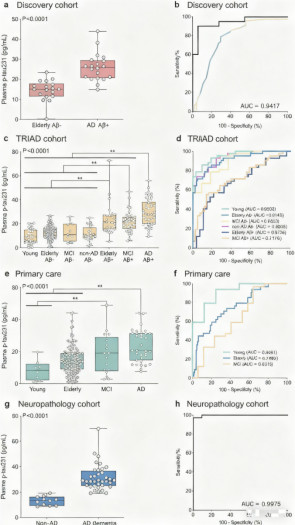
Discovery Cohort (n=38): Included AD patients (n=20) and age-matched controls (n=18), used for preliminary validation of the assay's ability to differentiate.
TRIAD Cohort (n=313): Comprised cognitively unimpaired young and older individuals, patients with Mild Cognitive Impairment, AD dementia patients, and non-AD dementia patients. All participants underwent CSF testing, Aβ-PET and tau-PET imaging, and detailed neuropsychological assessment.
Primary Care Cohort (n=190): Represented individuals from a primary care setting who had not been evaluated in specialized memory clinics, used to test the biomarker's diagnostic performance in a broader population.
Neuropathology Cohort (n=47): Included post-mortem, neuropathologically confirmed AD (n=36) and non-AD dementia (n=11) cases, for whom plasma samples had been collected 1-9 years before death. This cohort aimed to validate the specificity of plasma p-tau231 for AD diagnosis against the gold standard and analyze its relationship with Braak neurofibrillary tangle stages.
III. How accurate is the diagnostic performance of plasma p-tau231?
Analyses across multiple independent cohorts consistently demonstrated that plasma p-tau231 exhibits excellent diagnostic value in differentiating AD.
In the Discovery Cohort, its area under the curve for diagnosing AD was as high as 0.94.
In the TRIAD Cohort, plasma p-tau231 concentrations were significantly higher in Aβ-positive cognitively unimpaired older adults, MCI patients, and AD dementia patients compared to the Aβ-negative control group. Notably, its performance in differentiating Aβ-positive cognitively unimpaired individuals from Aβ-negative individuals surpassed that of another widely studied biomarker, plasma p-tau181.
In the Neuropathology Cohort, plasma p-tau231 could distinguish AD from non-AD neurodegenerative diseases with near-perfect accuracy (AUC = 0.997). Furthermore, its concentration progressively and significantly increased with advancing Braak stages (from I-II to V-VI), demonstrating a strong correspondence with the severity of AD neuropathology.
IV. How is plasma p-tau231 associated with brain pathology?
To investigate the relationship between plasma p-tau231 and core brain pathologies, correlation analyses were conducted:
Correlation with Tau Pathology: Plasma p-tau231 concentration showed a strong positive correlation with the extent of tangle deposition in the cerebral cortex (particularly the temporal lobe and cingulate cortex), as measured by tau-PET imaging.
Correlation with Aβ Pathology: Plasma p-tau231 was also significantly correlated with the burden of cortical Aβ plaque deposition, as measured by Aβ-PET, especially in the precuneus, frontal cortex, and striatum. A key finding was that this correlation persisted even in cognitively unimpaired Aβ-negative individuals, suggesting that p-tau231 changes might begin at the very early stages of Aβ accumulation.
V. Does plasma p-tau231 have early warning value?
One of the most significant findings of this study is that plasma p-tau231 shows abnormal elevation earlier than other biomarkers.
Earlier than Aβ-PET Positivity Threshold: Model analysis revealed that the inflection point for the increase in plasma p-tau231 occurs before Aβ deposition reaches the PET-positive diagnostic threshold. This suggests that p-tau231 begins to respond to minimal Aβ pathology during the subclinical stage, before an individual is classified as 'positive' by conventional Aβ-PET scans.
Earlier than Other Phosphorylated Tau Biomarkers: When subjects were grouped by Aβ deposition load, plasma p-tau231 was abnormally elevated in groups with lower Aβ load, whereas plasma p-tau181 and CSF p-tau217 showed abnormalities only in groups with higher Aβ load. This establishes the early position of p-tau231 in the temporal sequence of AD biomarker changes.
Sensitive to Early Tau Deposition: Plasma p-tau231 effectively differentiated Braak stage I-II from later tau pathology stages, whereas plasma p-tau181 showed no significant difference in this regard. This suggests that p-tau231 has a unique advantage in detecting the earliest neurofibrillary tangle deposition.
VI. Conclusions and Outlook: What is the clinical application prospect of plasma p-tau231?
Plasma p-tau231 is a highly promising biomarker for the early pathology of AD. Its application has the potential to revolutionize AD clinical practice, particularly in early screening and clinical trial enrollment. By identifying individuals at very high risk who are in the 'sprouting stage' of Aβ pathology or have only entorhinal tau deposition, plasma p-tau231 testing can facilitate ultra-early intervention for AD, securing a critical time window for treatment strategies targeting the root causes of the disease.
VII. Which manufacturers provide p-tau231 antibodies?
Hangzhou Start Bio-tech Co., Ltd.'s self-developed "Tau (phospho T231) Mouse Monoclonal Antibody" is a key reagent for neurobiological research, characterized by excellent phosphorylation specificity, high affinity, and superior stability. This product is ideal for studying early pathological events, disease diagnosis, and drug development for Alzheimer's disease and other tauopathies.
Product Core Advantages:
Excellent Phosphorylation Specificity & High Affinity: Highly specific for Tau pT231, effectively detecting endogenous levels in applications like Western Blot and IHC.
Excellent Stability & Batch Consistency: Ensures reliable and reproducible data in long-term studies.
Suitable Key Application Scenarios:
This product is an ideal tool for:
Early Pathological Detection in Alzheimer's Disease: Detects the early phosphorylation event pT231.
Neuropathological Diagnosis & Differentiation: Aids IHC staining for phosphorylated Tau in patient brain tissue sections.
Research on Tau Phosphorylation Mechanisms: Studies kinase/phosphatase regulation of the T231 site.
Drug Screening & Efficacy Evaluation: Serves as a key tool antibody for screening modulators of the T231 phosphorylation pathway.
Professional Technical Support: We provide detailed technical documentation and specialized technical consultation to assist customers in achieving precise and reliable discoveries in neurodegenerative disease research.
Hangzhou Start Bio-tech Co., Ltd. is committed to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details or to request a sample test, please contact us.
Product Information
| Catalog Number | Product Name | Product Parameters |
| S0B0054 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-1) |
Host : Rabbit |
| S0B3216 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-17) |
Host : Rabbit Conjugation : Unconjugated |
| S0B3217 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-1) |
Host : Rabbit Conjugation : Unconjugated |
| S0B3220 | Tau (phospho T231) Mouse mAb (SDT-202-2) | Host : Mouse Conjugation : Unconjugated |
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Tahoe Therapeutics generates the largest single-cell atlas ever using INTEGRA Bi 2/14/2026
- Evaluating the Clinical Value of Chromogranin A Antibodies in Neuroendocrine Tum 2/13/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026


