Home > News > MetP® Technology Unlocks Intranasal Delivery of Semaglutide and Other Brain-Targeting Peptides
MetP® Technology Unlocks Intranasal Delivery of Semaglutide and Other Brain-Targeting Peptides
- Enabling efficient nose-to-brain drug delivery, MetP® offers an alternative to injection-based administration - targeting a $150 billion obesity and neurological market.
EMMETTEN, Switzerland, July 08, 2025 / Biotech Newswire / -- MetP Pharma AG, a pioneer in nose-to-brain drug delivery for nearly two decades, announces new data highlighting the advantages of its proprietary MetP® Technology in delivering neuroactive peptides such as semaglutide directly to the brain via the intranasal route.
In a pharmaceutical landscape facing declining prices, expiring patents, and disrupted supply chains, reformulating existing drugs for intranasal delivery presents a timely innovation opportunity. With semaglutide as a reference compound, MetP® demonstrates that its platform can significantly enhance brain uptake while minimizing systemic exposure - addressing both efficacy and safety.
“Delivering peptides to the brain is notoriously difficult due to their molecular properties and formulation challenges,” said Dr. Claudia Mattern, Chief Scientific Officer of MetP Pharma. “The MetP® Technology overcomes these barriers by leveraging perineural and perivascular transport pathways, bypassing the blood-brain barrier and enabling direct, efficient brain delivery.”
Current subcutaneous (SC) and oral formulations of semaglutide, widely used in weight-loss and diabetes care, are limited by poor oral bioavailability, gastrointestinal side effects, inconvenient administration, and low central nervous system (CNS) penetration.
By contrast, MetP®’s intranasal formulation significantly enhances CNS penetration, a critical prerequisite for the brain to effectively mediate semaglutide’s weight-loss effects and a potential paradigm shift in the treatment of obesity and diabetes. Studies show that the MetP® Technology enables:
Rapid brain uptake (≤ 1 hour)
Sustained CNS exposure (≥ 24 hours)
High brain-to-plasma ratio, superior to SC, oral and IV delivery
Minimal systemic exposure, potentially reducing peripheral side effects
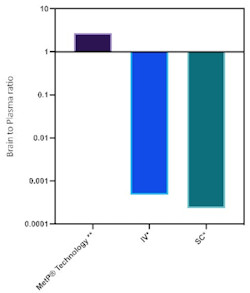
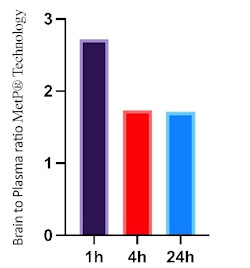
Figure 2 demonstrates the durability of brain exposure, showing brain-to-plasma ratios at 1, 4 and 24 hours post-administration with MetP® Technology, confirming its potential for sustained CNS availability over time.
"Conventional semaglutide delivery results in high plasma levels but minimal brain availability, which may contribute to side effects like muscle loss,” said Dr. Mattern. “Our data suggest that MetP® enabled intranasal delivery could not only improve CNS targeting but also unlock broader clinical applications beyond obesity.”
The implications of this technology extend far beyond semaglutide. With no active transport mechanism currently available to efficiently deliver peptides into the brain, MetP® may serve as a platform for a wide range of neuroactive therapeutics - from metabolic to neurodegenerative diseases.
Link to the press release
About MetP® Pharma AG
MetP® Pharma AG is an independent pharmaceutical R&D and product development company based in Switzerland, specializing in nose-to-brain drug delivery. The company’s proprietary MetP® Technology is a comprehensively patented platform that enables direct, efficient delivery of therapeutics to the brain, bypassing the blood-brain barrier.
The brain-targeting capabilities of MetP® Technology have been validated by several independent research institutions worldwide and are featured in 67 predominantly peer-reviewed publications. The platform has proven its clinical utility in several preclinical and clinical trials and has demonstrated to be fast, safe, effective, user-friendly, and cost-efficient.
MetP® is actively advancing its own pipeline of intranasal therapeutics targeting conditions such as concussion, insomnia, ADHD, multiple sclerosis (MS), and hypogonadism. A strong global intellectual property portfolio protects the platform until 2037/2039.
Beyond formulation, MetP® offers an integrated, end-to-end solution including its proprietary unit-dose applicator and industrial-scale filling technology - providing partners with a fully developed, patent-protected, and ready-to-use drug delivery system.
Contact
MetP Pharma AG
Dr. Claudia Mattern
CSO
+41-41-618 30 30
info@mattern-pharma.com
Reference:
(1) Lee TS et al. Novel LC-MS/MS analysis of the GLP-1 analog semaglutide with its application to pharmacokinetics and brain distribution studies in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Apr 15;1221:123688. doi: 10.1016/j.jchromb.2023.123688. Epub 2023 Mar 22. PMID: 36989942.
Keywords: Blood-Brain Barrier; semaglutide; Brain; Peptides; Obesity; Diabetes Mellitus; Technology; Pharmaceutical Preparations; Switzerland; MetP® Technology; MetP Pharma AG; intranasal delivery; nose-to-brain drug delivery; neuroactive peptides; brain targeting; CNS penetration; perineural transport; perivascular transport; blood-brain barrier bypass; rapid brain uptake; sustained CNS exposure; minimal systemic exposure; Dr. Claudia Mattern; formulation challenges; reformulation of existing drugs
Source: Biotech Newswire
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- CD146 Antibodies: Targeting Lipid Metabolism and Energy Homeostasis to Intervene 1/23/2026
- Be Prepared for 2026 Weather Extremes Says Cold Chain Technologies 1/23/2026
- MetP Pharma’s Enabling Technology Creates a New Brain-Targeted GLP-1 Opportunity 1/23/2026
- INTEGRA launches lab evolution automation competition 1/22/2026
- SP263: A Key Tool in Immunohistochemical Labeling 1/22/2026
- Quoin Pharmaceuticals Files Breakthrough Medicine Designation Application in Sau 1/21/2026
- Neurizon Secures Global Trademark Protection Across Key Markets 1/21/2026
- CD170 Antibody: How to Block the Tumor Immune Escape Mechanism Mediated by Myelo 1/21/2026
- Effect of iPS Cell Culture Medium on Differentiation Efficiency 1/21/2026


