Home > News > 8th Asian Biosimilars Congress, Aug 2017, Beijing China
8th Asian Biosimilars Congress, Aug 2017, Beijing China
- Directors, CEO’s of Organizations
- Business Development Managers
- Chief Scientific Officers
- R&D Researchers from Biosimilar and Biologics Industries
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Patent Attorneys
- Intellectual Property Attorneys
- Investment Analysts
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneurs
- Industry 60%
- Academia 30%
- Others 10%
- The resources and infrastructure are already in place in these countries.
- The current industry infrastructure, investment incentives and regulatory framework, and key participants in India, Singapore, Korea and China are promising.
- In addition, speakers’ profiled examples of companies are already building their biologics capabilities in these Asian countries.
- Asia can be a launch pad for biosimilars because of one, supportive governments.
- And lastly, these countries have a developed clinical infrastructure.
- South Korea was the first country worldwide to approve biosimilar versions of etanercept according to international standards. In addition, the country’s regulators are currently working on class-specific biosimilar regulatory guidelines.
- Australia, as of August 2016, has approved 11 biosimilars (based on six originators) – second only to the EU. Australia is the world’s first highly regulated market to allow pharmacy level substitution of a monoclonal antibody biosimilar for an originator.
- As of April 2016, eight biosimilars have been approved in Japan, including two insulin glargine biosimilars.
- A recent report has shown that biosimilars in India have witnessed nearly 20% annual growth for the last financial year and now make up for about 2.5% of the overall biologics market.
- In Japan and South Korea, formal price discount requirements for biosimilars generally range from 30–50% compared with the originator product.
- In South Korea, biosimilar competition is also driving down the price of originator products, with the price of the original reference product automatically dropping to 70% of its original market price as soon as the first biosimilar product is introduced into the market.
- Price reductions of more than one-third of the originator price have been seen with the introduction of recent biosimilars for rheumatoid arthritis in India.
- In China, a biosimilar to insulin glargine was introduced in 2013 (prior to the introduction of China’s official guidelines) with a price reduction of 26% compared with the originator.
8th Asian Biosimilars Congress (Biosimilars Asia 2017)
Theme: Biosimilars- Uncovering an innovation in motion
Dates: August 10-12, 2017
Venue: Beijing, China
Registration Link: http://biosimilars-biologics.pharmaceuticalconferences.com/asiapacific/registration.php
8th Biosimilars Congress 2017 Organizing Committee invites you to attend the largest assemblage of biologics and biosimilars researchers from around the globe during August 10-12, 2017 at Beijing, China.
Asian Biosimilars Congress is a global annual event. This Biosimilars Congress 2017 brings together scientists, researchers, business development managers, CEOs, directors, IP Attorneys, Regulatory Officials and CROs from around the world. The passage of biosimilars through a decade at Asia finds much requirement for discussion also focusing the latest developments in the field of biologics and biosimilars.
Why to attend?
Join your peers around the world focused on learning about Biologics and Biosimilars related advances, which is your single best opportunity to reach the largest assemblage of participants from the Biosimilars community, conduct demonstrations, distribute information, meet with current and potential professionals, make a splash with a new research works, and receive name recognition at this 3-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Biologics and Biosimilars are hallmarks of this conference.
Target Audience:
Audience Share:
Session/Tracks
Track 1:Latest Biosimilars in Asian Scenario
Track 2:Biosimilars- Hatch-Waxman Act & BPCI Act
Track 3:Challenges in Developing Biosimilars
Track 4:Biosimilars Research Pipeline
Track 5:Current Developments in The Field of Biosimilars
Track 6:Pharmacovigilance of Biosimilars
Track 7:Emerging Trends in Biosimilars
Track 8:Biosimilars Market- Challenges & Prospects
Track 9:Cost Analysis of Biosimilars
Track 10:Globalization of Biosimilars
Track 11:Biosimilars Analytical Strategies
Track 12:Emerging Biosimilars in Therapeutics
Track 13:Clinical Studies on Biosimilars
Track 14:BCS & IVIVC Based Biowaivers
Track 15:Entrepreneurs Investment Meet
Track 2:Biosimilars- Hatch-Waxman Act & BPCI Act
Track 3:Challenges in Developing Biosimilars
Track 4:Biosimilars Research Pipeline
Track 5:Current Developments in The Field of Biosimilars
Track 6:Pharmacovigilance of Biosimilars
Track 7:Emerging Trends in Biosimilars
Track 8:Biosimilars Market- Challenges & Prospects
Track 9:Cost Analysis of Biosimilars
Track 10:Globalization of Biosimilars
Track 11:Biosimilars Analytical Strategies
Track 12:Emerging Biosimilars in Therapeutics
Track 13:Clinical Studies on Biosimilars
Track 14:BCS & IVIVC Based Biowaivers
Track 15:Entrepreneurs Investment Meet
Market Analysis
The global Biosimilars market (Follow-On-Biologics) is expected to reach $26.5Billion by 2020 growing at a CAGR of 49.1% from 2015 to 2020 whereas The global biosimilars market alone is expected to reach $6.22 Billion by 2020 from $2.29 Billion in 2015, at a compound annual growth rate (CAGR) of 22.1% from 2015 to 2020.
Geographically, the global biosimilars market is dominated by Europe, followed by Asia-Pacific, Rest of the World (RoW), and North America. However, the Asia-Pacific region is likely to witness the highest growth rate during the forecast period.
With the biosimilar regulatory pathway in sight in markets around the world, companies from the biopharma innovators to traditional small molecule generic houses to well-funded start-ups are exploring how they can enter the biosimilar business.
Countries in Asia, particularly India, Singapore, Korea, Japan and China, are the attractive locations to build bio development and bio manufacturing capabilities.
Benefits for establishing Biosimilar Companies in Asia:
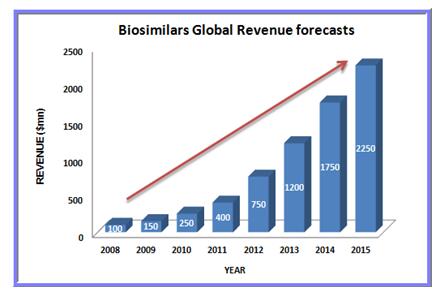
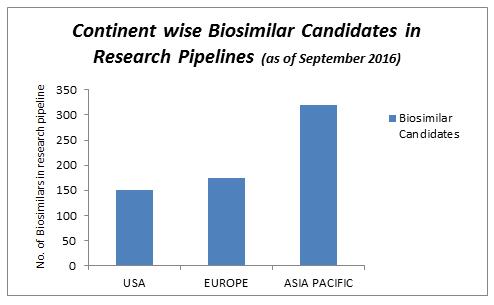
There are currently more biosimilar products in development across the Asia-Pacific region than anywhere else in the world, leading to a wealth of opportunities for investigators and patients to take part in biosimilar clinical trials.
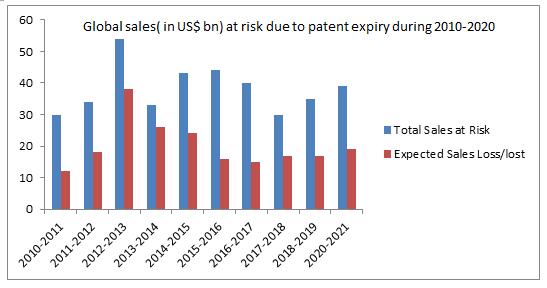
Substantial price reductions for biosimilars have been seen within the Asia-Pacific region:
A number of factors such as growing pressure to curtail healthcare expenditure, growing demand of biosimilar drugs due to their cost effectiveness, rising incidences of various diseases, increasing number of off-patented drugs, positive outcome in the ongoing clinical trials, and rising demand for biosimilars in different therapeutic applications such as rheumatoid arthritis and blood disorders are propelling the growth of the global market.
About City
Beijing (formerly Romanized as Peking) is the capital of the People's Republic of China and the world's third most populous city proper. It is also one of the world's most populous capital cities. Beijing is the second largest Chinese city by urban population after Shanghai and is the nation's political, cultural, and educational center. It is home to the headquarters of most of China's largest state-owned companies, and is a major hub for the national highway, expressway, railway, and high-speed rail networks. The Beijing Capital International Airport is the second busiest in the world by passenger traffic. The city's subway network is among the longest and busiest in the world. The city is renowned for its opulent palaces, temples, parks, gardens, tombs, walls and gates, and its art treasures and universities have made it a center of culture and art in China.
Beijing has seven UNESCO World Heritage Sites – the Forbidden City, Temple of Heaven, Summer Palace, Ming Tombs, Zhoukoudian, as well as parts of the Great Wall and the Grand Canal. Beijing hosted the 2008 Summer Olympics and was chosen to host the 2022 Winter Olympics, which will make it the first city to ever host both events.
Drop us an email for Program enquiry.
Sponsors / Exhibiting / Advertising.
General Queries.
Related News
- CPHI & PMEC China 2026: Revolutionizing Laboratory Innovation in Asia 2/13/2026
- 2026 World Radiology and Medical Imaging Conference (2026WRMI) 2/12/2026
- Pharma Partnering EU Summit 2026 2/11/2026
- Pharma Partnering US Summit 2026 2/11/2026
- BioAsia 2026 to Convene the World’s Leading Science, AI, and Industry Pioneers D 2/5/2026
- Global Medical Expo 2026 1/29/2026
- GENAP Summit 2026 1/21/2026
- LAB INDONESIA 2026 1/16/2026
- Cold Spring Harbor Asia Conference | Illuminating the Brain–a Symposium on Neura 1/13/2026
- Cold Spring Harbor Asia Conference | Autism & Neurodevelopment Disorders 1/13/2026



