Home > News > Optimizing Colorectal Cancer Treatment: CD43 Antibodies Remodel the Tumor Immune Microenvironment
Optimizing Colorectal Cancer Treatment: CD43 Antibodies Remodel the Tumor Immune Microenvironment
- 1. Concept
Colorectal cancer (CRC) is one of the most prevalent gastrointestinal malignancies globally, yet its treatment faces significant bottlenecks. Current immunotherapies, such as immune checkpoint inhibitors, only benefit approximately 14% of patients with high microsatellite instability (MSI-H)—a subgroup accounting for merely 5% of all CRC cases. Most patients exhibit poor responsiveness to existing regimens, while chemotherapy is limited by toxicity, drug resistance, and high recurrence rates. CD43, a leukocyte surface molecule, has emerged as a promising therapeutic target: targeting CD43 on tumor cells reshapes the tumor immune microenvironment (TIME), enhances anti-tumor immune responses, and exhibits synergistic effects with existing immunotherapies—offering a new avenue to overcome treatment limitations in CRC.
2. Research Frontiers
2.1 Clinical Challenges in Colorectal Cancer Immunotherapy
CRC immunotherapy is hindered by several critical barriers:
Limited Responsiveness: The majority of CRC patients have microsatellite-stable (MSS) tumors, which are inherently resistant to immune checkpoint inhibitors, leaving most patients without effective immunotherapeutic options.
Tumor Immune Evasion: The TIME in CRC is often immunosuppressive, characterized by reduced infiltration of effector T cells, increased presence of immunosuppressive cells (e.g., regulatory T cells, myeloid-derived suppressor cells), and impaired antigen presentation—enabling tumors to evade immune surveillance.
Chemotherapy Limitations: While chemotherapy can induce immunogenic cell death, its clinical benefits are constrained by systemic toxicity, acquired drug resistance, and failure to fundamentally reverse the immunosuppressive TIME.
These challenges underscore the urgent need for novel targets and strategies to activate anti-tumor immunity in CRC.
2.2 The Role of CD43 in Colorectal Cancer Immune Regulation
Recent preclinical studies have identified CD43 as a key regulator of CRC immunity:
Tumor Growth Inhibition: Specific targeting of CD43 on CRC tumor cells significantly suppresses tumor progression in mouse models, demonstrating its potential as a therapeutic target.
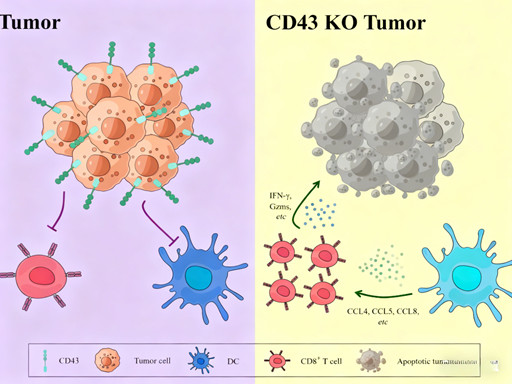
Immune Modulation: CD43 deficiency in tumor cells enhances infiltration of diverse immune cells (e.g., CD8⁺ T cells, dendritic cells) into the TIME and strengthens long-term immune memory. This effect is primarily T cell-dependent, highlighting CD43’s role in regulating anti-tumor immune responses and reversing immune evasion.
2.3 Specific Mechanisms of CD43-Mediated Tumor Immune Microenvironment Remodeling
CD43 regulates the TIME through multi-layered, cell-specific mechanisms:
Cellular Level Regulation: Targeting CD43 enhances CD8⁺ T cell-mediated cytotoxicity, promotes the activation and maturation of antigen-presenting cells (APCs) such as dendritic cells (DCs), and improves T cell chemotaxis into the tumor core—addressing the "cold tumor" phenotype common in MSS CRC.
Molecular Level Regulation: CD43 deficiency alters the interaction landscape between tumor cells and immune cells, reducing the expression of immunosuppressive molecules (e.g., PD-L1, TGF-β) and increasing the secretion of pro-inflammatory cytokines (e.g., IFN-γ, granzyme B). This reshapes the TIME into a pro-immune state conducive to anti-tumor responses.
Cell-Specificity: Critically, CD43’s regulatory effects are primarily mediated by targeting CD43 on tumor cells themselves, rather than modulating CD43 expression in host immune cells—minimizing potential off-target effects on healthy immune function.
2.4 Synergistic Effects Between CD43-Targeted Therapy and Existing Immunotherapies
A key breakthrough in CD43 research is its synergistic potential with immune checkpoint inhibitors:
Sensitization to PD-L1 Blockade: CRC models with CD43-deficient tumor cells show significantly increased sensitivity to PD-L1 inhibitors. CD43 targeting "primes" the TIME by enhancing T cell infiltration and activation, converting immunosuppressive "cold tumors" into immunoresponsive "hot tumors" that are susceptible to checkpoint blockade.
Mechanistic Synergy: CD43-targeted therapy remodels the TIME to enhance T cell access and function, while PD-1/PD-L1 inhibitors relieve T cell exhaustion—acting through complementary pathways to amplify anti-tumor immunity. This combination strategy has the potential to expand the beneficiary population beyond MSI-H patients to include MSS CRC patients.
2.5 Clinical Translation Potential of CD43-Targeted Therapy
CD43-targeted therapy holds strong promise for clinical translation in CRC:
Specific Targeting: CD43 exhibits tumor-specific expression patterns in CRC, providing a clear therapeutic window to minimize damage to healthy tissues.
Broad Applicability: Preclinical data suggest CD43 targeting may benefit multiple CRC molecular subtypes, including MSS tumors—addressing the unmet need for effective immunotherapies in the majority of patients.
Combination Versatility: Synergy with existing immunotherapies (e.g., PD-1/PD-L1 inhibitors), chemotherapy, or targeted agents (e.g., EGFR inhibitors) offers flexible treatment design and the potential to improve response rates and survival.
2.6 Future Research Directions
To advance CD43-targeted therapy toward clinical application, key research priorities include:
Elucidating Signaling Pathways: Defining the detailed molecular signaling cascades through which CD43 regulates the TIME, including downstream targets and interaction partners.
Addressing Expression Heterogeneity: Investigating CD43 expression patterns across CRC subtypes, stages, and patient populations to identify optimal candidates for targeted therapy.
Optimizing Combination Strategies: Exploring the timing, dosage, and sequencing of CD43-targeted therapy with other agents to maximize synergy and minimize toxicity.
Developing Precision Tools: Creating sensitive and specific diagnostic assays to detect CD43 expression in tumors, enabling patient stratification and treatment monitoring.
Clinical Safety and Efficacy Evaluation: Conducting early-phase clinical trials to assess the safety, pharmacokinetics, and preliminary efficacy of CD43-targeted antibodies in CRC patients.
3. Research Significance
CD43-targeted therapy addresses a critical gap in CRC treatment by targeting the root cause of immunotherapy resistance: the immunosuppressive tumor immune microenvironment. By reshaping the TIME, enhancing T cell function, and promoting antigen presentation, CD43 offers a novel strategy to convert non-responsive tumors into treatable ones. Its synergistic potential with immune checkpoint inhibitors could expand the reach of immunotherapy to the vast majority of MSS CRC patients, significantly improving overall treatment outcomes. Additionally, CD43’s role as a leukocyte surface marker provides diagnostic value for T-cell lymphomas and immune-related diseases, highlighting its multifaceted utility in cancer research and clinical practice.
4. Related Mechanisms, Research Methods, and Product Applications
4.1 Mechanisms
CD43-mediated TIME remodeling is driven by: (1) Enhancement of T cell cytotoxicity and infiltration; (2) Activation of antigen-presenting cells; (3) Downregulation of immunosuppressive molecules and upregulation of pro-inflammatory cytokines; (4) Synergistic relief of T cell exhaustion when combined with checkpoint inhibitors. These mechanisms collectively reverse immune evasion and amplify anti-tumor immunity.
4.2 Research Methods
Key research methods for studying CD43 in CRC include:
Immunohistochemistry (IHC) and Immunofluorescence: Detect CD43 expression in tumor tissues and assess immune cell infiltration (e.g., CD8⁺ T cells, DCs) in the TIME.
Flow Cytometry: Quantify CD43 expression on tumor cells and immune subsets, and evaluate T cell activation markers (e.g., CD69, CD25) and cytotoxic function.
In Vitro Functional Assays: Measure T cell-mediated cytotoxicity, DC maturation, and cytokine secretion (e.g., IFN-γ, TNF-α) in co-cultures of CD43-modified tumor cells and immune cells.
In Vivo Models: Use CRC xenograft models or genetically engineered mouse models to evaluate tumor growth, survival, and immune responses following CD43 targeting or combination therapy.
Molecular Profiling: RNA sequencing (RNA-seq) and proteomics to identify CD43-regulated genes and pathways in the TIME.
4.3 Product Applications
ANT BIO PTE. LTD.’s CD43 antibodies, anchored by the STARTER brand’s "S-RMab® CD43 Recombinant Rabbit Monoclonal Antibody" (Catalog No.: S0B2144), are essential tools for CRC research and clinical diagnostics:
T-Cell Lymphoma Diagnosis: Enables accurate diagnosis and differential diagnosis of peripheral T-cell lymphoma, mycosis fungoides, and other T-cell malignancies through specific CD43 detection.
Lymphocyte Activation Studies: Facilitates investigation of CD43 expression dynamics and functional roles during T cell activation—critical for understanding anti-tumor immune responses.
Hematopoietic Cell Differentiation Analysis: Supports expression profiling of CD43 during myeloid and lymphocyte differentiation, advancing hematology research.
Inflammation and Immune Disease Research: Aids in detecting and analyzing activated lymphocytes in autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis) and inflammatory conditions.
Colorectal Cancer Research: Validated for IHC, the S0B2144 antibody enables CD43 expression analysis in CRC tissues, supporting TIME research and preclinical development of CD43-targeted therapies.
The S0B2144 antibody, developed using ANT BIO PTE. LTD.’s proprietary S-RMab® recombinant rabbit monoclonal platform, offers exceptional advantages: high specificity with clear membrane localization (ensuring reliable detection in FFPE samples) and superior staining stability with minimal batch variation—critical for consistent results in clinical diagnostics and translational research.
5. Brand Mission
ANT BIO PTE. LTD. is dedicated to empowering the global life science community with high-quality, innovative biological reagents and solutions. Leveraging advanced development platforms—including recombinant rabbit monoclonal antibody, recombinant mouse monoclonal antibody, rapid monoclonal antibody, and multi-system recombinant protein expression platforms (E.coli, CHO, HEK293, Insect Cells)—and adhering to rigorous international certifications (EU 98/79/EC, ISO9001, ISO13485), we strive to deliver reliable, performance-proven tools that accelerate scientific breakthroughs in oncology, immunology, and hematology. Our commitment to quality and innovation aims to support researchers and clinicians in advancing human health through cutting-edge life science research and clinical translation.
6. Related Product List
| Catalog No. | Product Name | Host |
| S0B2144 | S-RMab® CD43 Recombinant Rabbit mAb (SDT-R101) | Rabbit |
| S0B1235 | Rat Anti-Mouse CD43 Antibody (S-R588) | Rat |
| S0B5285 | FITC Mouse Anti-Rat CD43 Antibody (S-R632) | Mouse |
| S0B8123 | Alexa Fluor® 647 Rat Anti-Mouse CD43 Antibody (S-R588) | Rat |
| S0B5692 | Alexa Fluor® 488 Rat Anti-Mouse CD43 Antibody (S-R588) | Rat |
7. AI Disclaimer
This article is AI-compiled and interpreted based on the original work. All intellectual property (e.g., images, data) of the original publication shall belong to the journal and the research team. For any infringement, please contact us promptly and we will take immediate action.
ANT BIO PTE. LTD. – Empowering Scientific Breakthroughs
At ANTBIO, we are committed to advancing life science research through high-quality, reliable reagents and comprehensive solutions. Our specialized sub-brands (Absin, Starter, UA) cover a full spectrum of research needs, from general reagents and kits to antibodies and recombinant proteins. With a focus on innovation, quality, and customer-centricity, we strive to be your trusted partner in unlocking scientific mysteries and driving medical progress. Explore our product portfolio today and elevate your research to new heights.
Related News
- Wnt3a Cytokine: A Multidimensional Exploration from Molecular Characteristics to 12/31/2026
- Unveiling the Multifaceted Value of CGA/HCG-α: From Pregnancy Monitoring to Dise 2/12/2026
- Azenta Life Sciences and Frontier Space Announce Strategic Partnership 2/12/2026
- Elucidating CD8α’s Core Mechanisms: Maintaining T Cell Homeostasis and Regulatin 2/11/2026
- GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital i 2/11/2026
- AnalytiChem to Showcase New Product Launches and its Wide-ranging Lab Solutions 2/11/2026
- CD79B Antibodies: Emerging as a Precise Target for B Cell-Related Disease Therap 2/10/2026
- Everest Medicines Announces China NMPA Approval of VELSIPITY(R) for Adults with 2/10/2026
- Precise Differential Diagnosis of Atypical Cells in Serous Cavity Effusions: The 2/9/2026
- Eppendorf Collaborates with Dubai Police to Automate Forensics Laboratories 2/9/2026


