|
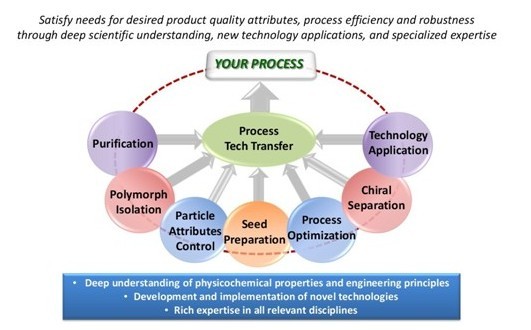
Crystallization Development
|
| Crystallization is a critical unit operation in API manufacturing. Desired product quality attributes can be obtained economically with high efficiency through well-designed and controlled crystallization processes. Successful crystallization development is based on a fundamental understanding of physicochemical and engineering principles, implementation and development of technologies and expertise in specialized areas. |
|
Purification
Crystallization is the most effective method to purify a chemical compound at industrial scale. A well-designed and controlled crystallization process >>
|
Process Optimization
Meeting delivery deadlines often takes higher priority than process optimization. As a drug candidate moves forward in development >>
|
|
Polymorphic Isolation
For a drug substance, a certain solid form is often desired for proper dissolution or bioavailability, stability, processing or intellectual property. >>
|
Technology Application
One of the highlights of our crystallization development is effective implementation of available and new technologies. In addition to our rich >>
|
|
Particle Attributes Control
Many attributes of API particles (e.g., size, density, morphology, hydroscopicity, static behavior, etc.) can impact the quality of a final drug product. >>
|
Technology Transfer
Seamless transfer of crystallization processes developed at Crystal Pharmatech is ensured based on our experience in successfully >>
|
|
Chiral Separation
More than 70% of drug candidates worldwide are chiral. Typically, for chiral API, only one stereoisomer is biologically active. >>
|
|
|

|
bio-equip.cn Crystal Pharmatech is the first technology-driven, China based contract research organization (CRO) that focuses on materials science and engineering for drug development. We partner with clients to ensure comprehensive solutions for their needs in solid-state research, crystallization process development, and preformulation studies. We guide clients in the discovery and selection of the optimal solid phase for drug development using all aspects of pre-formulation studies, including API process and formulation development, regulatory support and intellectual property protection.
Founded in 2010, Crystal Pharmatech has business relationships with over 60 global pharmaceutical companies. High quality service, a culture of confidentiality, and fast turnaround with cost-effective pricing are our trademarks. As we continue to grow, Crystal Pharmatech will always keep customers our number one priority. We will also continue to be at the forefront of innovation in the field of solid-state chemistry so that our customers can reap the benefits of key discoveries in an ever changing field.
Our Scientific Advisory Board includes global leaders in drug development covering amorphous dispersions, co-crystals, crystallization, solid forms, formulation and pre-formulation. The role of our board is all encompassing while we search for new scientific areas for internal and external projects.
|
|
|