|
CTLA-4 Antibodies: How to Achieve a Breakthrough in Balancing Efficacy and Safety?
hits:45 Date:11/11/25
I. What Role Does CTLA-4 Play in Immune Regulation?
CTLA-4 (Cytotoxic T-Lymphocyte-Associated Protein 4) is a member of the immunoglobulin superfamily expressed on the surface of activated T cells. As a key immune checkpoint molecule, its primary function is to transmit inhibitory signals, regulating the degree of T cell activation. While this mechanism helps prevent autoimmune reactions, it can also be exploited by tumor cells to achieve immune escape.
From a molecular mechanism perspective, CTLA-4 competitively binds to B7 molecules (including B7-1 and B7-2) on the surface of antigen-presenting cells against the T cell co-stimulatory molecule CD28. Its affinity for B7 molecules is significantly higher than that of CD28, thereby effectively inhibiting T cell proliferation and activation. This characteristic makes it an important target for cancer immunotherapy.
II. What Challenges Does CTLA-4 Antibody Therapy Face?
Although CTLA-4 antibodies show potential in cancer treatment, their clinical application faces significant safety challenges. Since CTLA-4 is an important molecule for maintaining the body's immune tolerance, completely blocking its function may lead to severe immune-related adverse events (irAEs). Research data indicate that when CTLA-4 antibodies are combined with PD-1/L1 antibodies, 50%-90% of patients experience grade 3-4 immune therapy-related side effects.
The mechanism behind these side effects is closely related to the physiological function of CTLA-4 in maintaining autoimmune balance. In preclinical models, deletion of the CTLA-4 gene leads to severe autoimmune diseases, suggesting the need for a careful balance between anti-tumor efficacy and autoimmune toxicity when developing CTLA-4-targeted therapies.
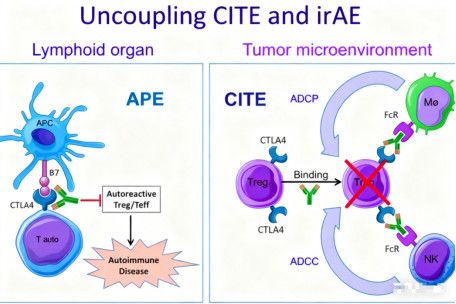
III. What Are the Innovative Mechanisms of Novel CTLA-4 Antibodies?
Newly developed CTLA-4 antibodies employ a unique mechanism of action, enabling selective depletion of tumor-infiltrating regulatory T cells (Tregs) while preserving the activation function of surrounding normal T cells. Unlike traditional CTLA-4 antibodies that promote CTLA-4 degradation in lysosomes, the novel antibodies maintain CTLA-4 recycling within cells, preserving its normal physiological function outside the tumor microenvironment.
This differential mechanism allows the novel antibody to effectively mediate targeted T cell depletion within the tumor while maintaining immune homeostasis in other parts of the body, thereby achieving an optimized balance between therapeutic effect and safety. Preclinical studies show that this antibody maintains potent anti-tumor activity while significantly reducing the risk of immune-related adverse events.
IV. How Does the Novel Antibody Achieve the Balance Between Safety and Efficacy?
Through precise mechanistic design, the novel CTLA-4 antibody decouples immune therapy-related side effects from anti-tumor efficacy. Research indicates that these two effects are mediated by different mechanisms, allowing for the engineering of antibody molecules with optimized properties through modification.
Specifically, the novel antibody effectively activates the anti-tumor function of T cells within the tumor microenvironment, promoting tumor-specific immune responses. In peripheral tissues, it maintains immune tolerance by preserving the normal function of CTLA-4. This tissue-specific regulatory capability gives it a better safety profile in clinical applications.
V. How Will Clinical Trials Validate Its Potential?
According to recent developments, the novel CTLA-4 antibody has received approval to proceed with clinical trials. The planned Phase I clinical trial will be conducted in two parts: Phase 1A will primarily evaluate the safety, pharmacokinetic profile, and preliminary efficacy of the antibody as a monotherapy in patients with advanced solid tumors. Phase 1B will explore its combination with PD-1/L1 inhibitors for treating non-small cell lung cancer (NSCLC).
This open-label trial is expected to systematically evaluate the performance of the novel antibody in humans, focusing on its ability to maintain efficacy while reducing toxicity. The trial results will provide critical evidence for the clinical application of this optimized CTLA-4 antibody.
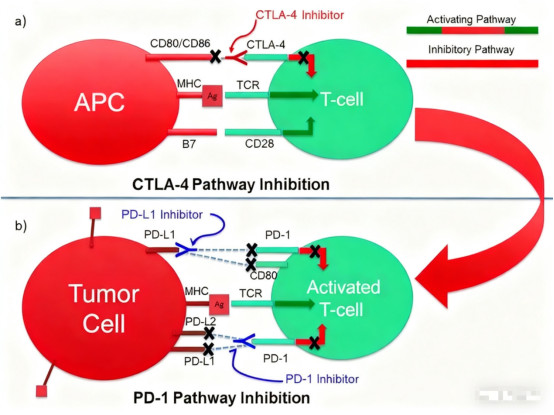
VI. What Are the Advantages of Combining CTLA-4 Antibodies with PD-1/L1 Inhibitors?
The CTLA-4 and PD-1/L1 pathways play complementary roles in immune regulation. CTLA-4 primarily regulates T cell activation during the initial phase of the immune response, while PD-1 mainly prevents excessive T cell activation during the effector phase. This mechanistic difference provides a theoretical basis for combination therapy.
Clinical studies show that the combination of CTLA-4 antibodies and PD-1/L1 inhibitors can produce synergistic effects, enhancing anti-tumor immune responses through different stages. In some tumor types, combination therapy demonstrates superior clinical outcomes compared to monotherapy, particularly in improving complete response rates.
VII. What is the Future Development Direction?
With a deeper understanding of CTLA-4's biological functions, the development of next-generation CTLA-4 antibodies will focus more on precisely regulating immune responses. Future research directions include further optimizing antibody specificity, developing tissue-targeted formulations, and exploring rational combinations with other immunotherapies.
Simultaneously, biomarker-based patient selection strategies will also become an important development direction. By identifying patient populations likely to benefit from CTLA-4 antibody therapy, personalized precision treatment can be achieved. As more clinical data accumulate, CTLA-4 antibodies are expected to play a more significant role in the field of cancer immunotherapy.
VIII. Which Companies Supply CTLA-4 Antibodies?
Hangzhou Starter Biotech Co., Ltd. (ANT BIO PTE. LTD. group member) has independently developed the "Human CTLA-4 Fc Chimera Protein" (Product Name: CTLA-4 Fc Chimera Protein, Human, Product Code: UA010039). This is a recombinant protein product characterized by high biological activity, high purity, and excellent stability. Produced using a mammalian cell expression system, it fuses the extracellular domain of human CTLA-4 with the human IgG Fc segment. It holds key application value in immune checkpoint mechanism research, drug screening and evaluation, and in vitro T cell function regulation studies.
Core Product Advantages:
* High Biological Activity and Specific Binding Capability: Validated by Surface Plasmon Resonance (SPR) and cell binding assays, this product efficiently binds its ligands CD80 (B7-1) and CD86 (B7-2), demonstrating biological activity similar to the native protein. Its Fc tag design facilitates immobilization operations and detection signal amplification.
* Excellent Purity and Stability: Through a multi-step chromatographic purification process, the product purity is >95% as analyzed by SDS-PAGE and SEC-HPLC, with endotoxin levels <1.0 EU/μg. Under stringent quality control standards, the product exhibits outstanding physicochemical stability and high batch-to-batch consistency, ensuring the accuracy and reproducibility of experimental data.
* Suitable for Various Key Application Scenarios: This product is an ideal tool for the following research areas:
Immune Checkpoint Blockade Research: Serves as a "decoy receptor" for CD80/CD86 to mimic an immunosuppressive environment and evaluate the inhibitory role of the CTLA-4 pathway in T cell activation.
Drug Screening and Evaluation: Used for screening and evaluating candidate drugs (e.g., antibodies, small molecule inhibitors) targeting the CTLA-4/CD80/CD86 interaction.
Protein Interaction and Signaling Pathway Research: Used to study the binding affinity, kinetics, and downstream signaling mechanisms of CTLA-4 with its ligands.
In Vitro T Cell Function Analysis: In T cell activation assay systems, used to specifically block B7 co-stimulatory signals and study its regulation of T cell proliferation and function.
* Professional Technical Support: We provide detailed product technical documentation, including complete purity and activity analysis reports, binding affinity data, and professional application solutions, fully committed to assisting customers in making progress in tumor immunology and drug development research.
Hangzhou Starter Biotech Co., Ltd. (ANT BIO PTE. LTD. group member) is consistently dedicated to providing high-quality, high-value biological reagents and solutions for global innovative pharmaceutical companies and research institutions. For more details about the "Human CTLA-4 Fc Chimera Protein" (Product Code UA010039) or to request a sample test, please feel free to contact us.
Product Information
| Catalog Number |
Product Number |
Product Parameters |
| UA011177 |
Biotinylated CTLA-4/CD152 His & Avi Tag Protein, Mouse |
Host : Mouse
Expression System : HEK293
Conjugation : Biotin |
| S0B0574 |
Invivo anti-mouse CTLA-4 (CD152) mAb |
Host : Mouse
Conjugation : Unconjugated |
| UA010784 |
CTLA-4/CD152 Fc Chimera Protein, Rat |
Host : Rat
Expression System : HEK293
Conjugation : Unconjugated |
| UA010792 |
CTLA-4/CD152 His Tag Protein, Rat |
Host : Rat
Expression System : HEK293
Conjugation : Unconjugated |
| UA010039 |
CTLA-4 Fc Chimera Protein, Human |
Host : Human
Expression System : HEK293
Conjugation : Unconjugated |
|